Abstract
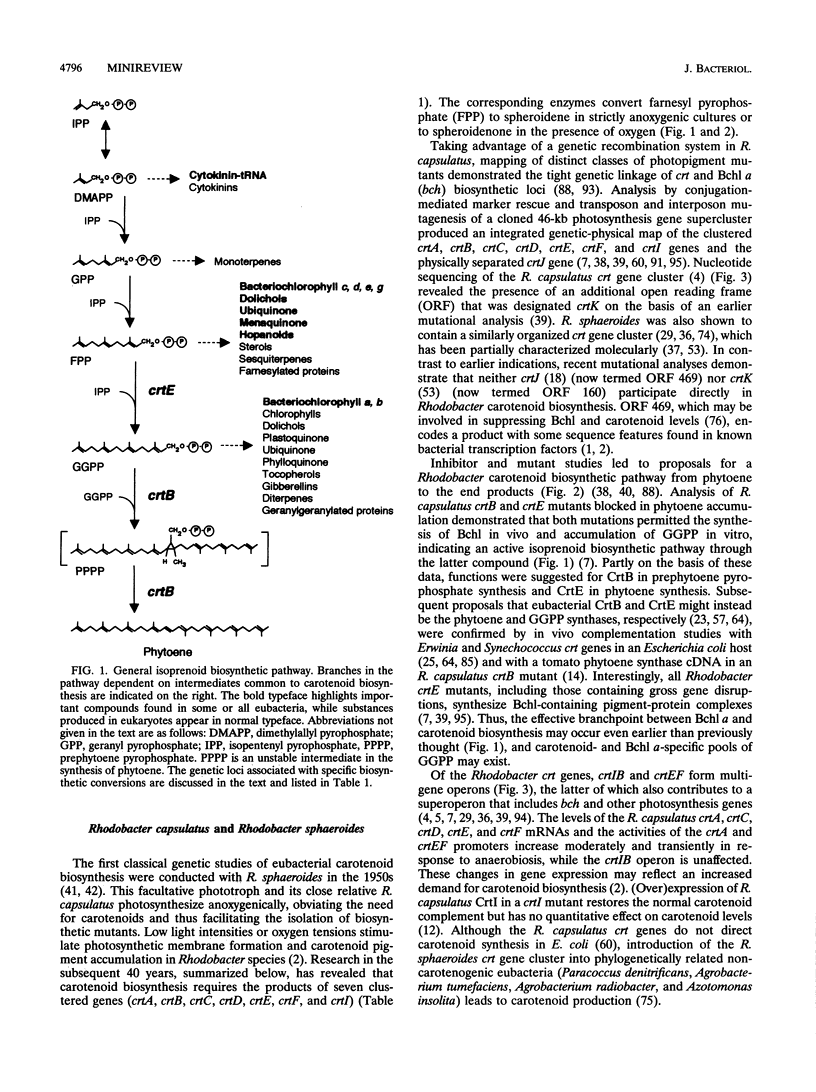
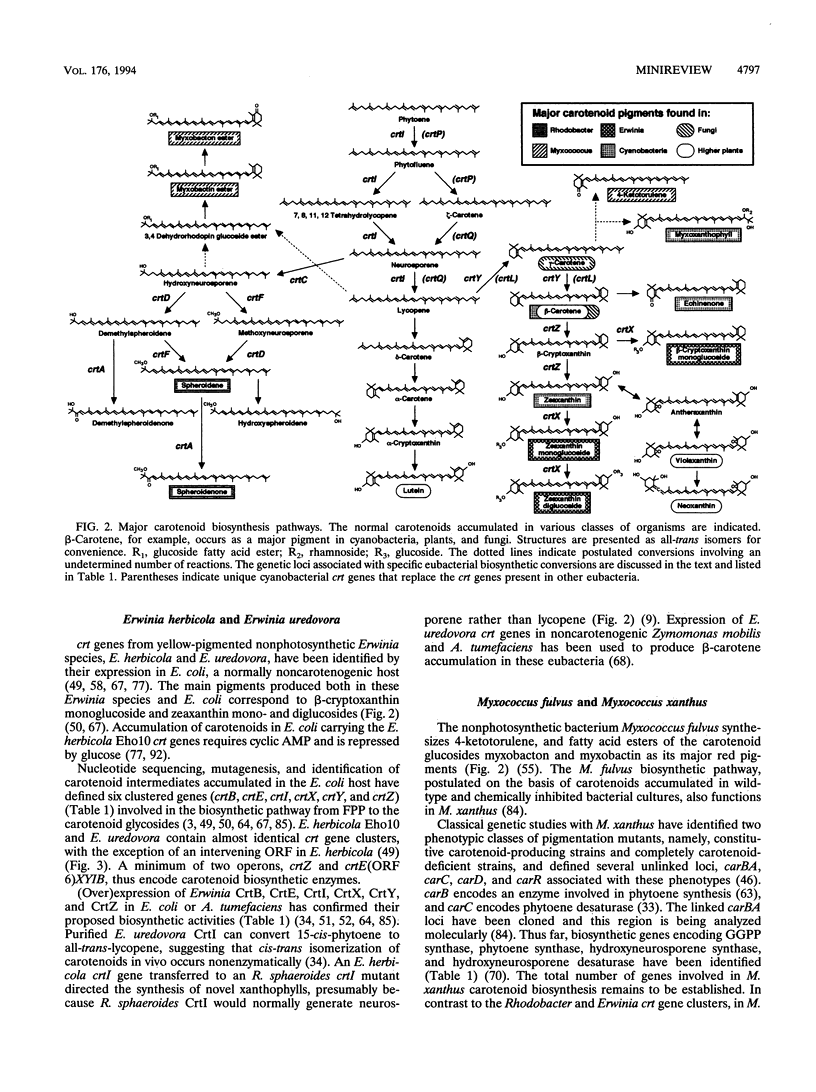
The opportunities to understand eubacterial carotenoid biosynthesis and apply the lessons learned in this field to eukaryotes have improved dramatically in the last several years. On the other hand, many questions remain. Although the pigments illustrated in Fig. 2 represent only a small fraction of the carotenoids found in nature, the characterization of eubacterial genes required for their biosynthesis has not yet been completed. Identifying those eukaryotic carotenoid biosynthetic mutants, genes, and enzymes that have no eubacterial counterparts will also prove essential for a full description of the biochemical pathways (81). Eubacterial crt gene regulation has not been studied in detail, with the notable exceptions of M. xanthus and R. capsulatus (5, 33, 39, 45, 46, 84). Determination of the rate-limiting reaction(s) in carotenoid biosynthesis has thus far yielded species-specific results (12, 27, 47, 69), and the mechanisms of many of the biochemical conversions remain obscure. Predicted characteristics of some carotenoid biosynthesis gene products await confirmation by studying the purified proteins. Despite these challenges, (over)expression of eubacterial or eukaryotic carotenoid genes in heterologous hosts has already created exciting possibilities for the directed manipulation of carotenoid levels and content. Such efforts could, for example, enhance the nutritional value of crop plants or yield microbial production of novel and desirable pigments. In the future, the functional compatibility of enzymes from different organisms will form a central theme in the genetic engineering of carotenoid pigment biosynthetic pathways.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong G. A., Alberti M., Hearst J. E. Conserved enzymes mediate the early reactions of carotenoid biosynthesis in nonphotosynthetic and photosynthetic prokaryotes. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9975–9979. doi: 10.1073/pnas.87.24.9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong G. A., Alberti M., Leach F., Hearst J. E. Nucleotide sequence, organization, and nature of the protein products of the carotenoid biosynthesis gene cluster of Rhodobacter capsulatus. Mol Gen Genet. 1989 Apr;216(2-3):254–268. doi: 10.1007/BF00334364. [DOI] [PubMed] [Google Scholar]
- Armstrong G. A., Cook D. N., Ma D., Alberti M., Burke D. H., Hearst J. E. Regulation of carotenoid and bacteriochlorophyll biosynthesis genes and identification of an evolutionarily conserved gene required for bacteriochlorophyll accumulation. J Gen Microbiol. 1993 May;139(5):897–906. doi: 10.1099/00221287-139-5-897. [DOI] [PubMed] [Google Scholar]
- Armstrong G. A., Hundle B. S., Hearst J. E. Evolutionary conservation and structural similarities of carotenoid biosynthesis gene products from photosynthetic and nonphotosynthetic organisms. Methods Enzymol. 1993;214:297–311. doi: 10.1016/0076-6879(93)14073-r. [DOI] [PubMed] [Google Scholar]
- Armstrong G. A., Schmidt A., Sandmann G., Hearst J. E. Genetic and biochemical characterization of carotenoid biosynthesis mutants of Rhodobacter capsulatus. J Biol Chem. 1990 May 15;265(14):8329–8338. [PubMed] [Google Scholar]
- Ashby M. N., Edwards P. A. Elucidation of the deficiency in two yeast coenzyme Q mutants. Characterization of the structural gene encoding hexaprenyl pyrophosphate synthetase. J Biol Chem. 1990 Aug 5;265(22):13157–13164. [PubMed] [Google Scholar]
- Balsalobre J. M., Ruiz-Vazquez R. M., Murillo F. J. Light induction of gene expression in Myxococcus xanthus. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2359–2362. doi: 10.1073/pnas.84.8.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley G. E., Schmidhauser T. J., Yanofsky C., Scolnik P. A. Carotenoid desaturases from Rhodobacter capsulatus and Neurospora crassa are structurally and functionally conserved and contain domains homologous to flavoprotein disulfide oxidoreductases. J Biol Chem. 1990 Sep 15;265(26):16020–16024. [PubMed] [Google Scholar]
- Bartley G. E., Scolnik P. A. Carotenoid biosynthesis in photosynthetic bacteria. Genetic characterization of the Rhodobacter capsulatus CrtI protein. J Biol Chem. 1989 Aug 5;264(22):13109–13113. [PubMed] [Google Scholar]
- Bartley G. E., Scolnik P. A. cDNA cloning, expression during development, and genome mapping of PSY2, a second tomato gene encoding phytoene synthase. J Biol Chem. 1993 Dec 5;268(34):25718–25721. [PubMed] [Google Scholar]
- Bartley G. E., Viitanen P. V., Bacot K. O., Scolnik P. A. A tomato gene expressed during fruit ripening encodes an enzyme of the carotenoid biosynthesis pathway. J Biol Chem. 1992 Mar 15;267(8):5036–5039. [PubMed] [Google Scholar]
- Bartley G. E., Viitanen P. V., Pecker I., Chamovitz D., Hirschberg J., Scolnik P. A. Molecular cloning and expression in photosynthetic bacteria of a soybean cDNA coding for phytoene desaturase, an enzyme of the carotenoid biosynthesis pathway. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6532–6536. doi: 10.1073/pnas.88.15.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C. E., Bollivar D. W., Suzuki J. Y. Genetic analyses of photopigment biosynthesis in eubacteria: a guiding light for algae and plants. J Bacteriol. 1993 Jul;175(13):3919–3925. doi: 10.1128/jb.175.13.3919-3925.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer P., Mayer M., Kleinig H. Molecular oxygen and the state of geometric isomerism of intermediates are essential in the carotene desaturation and cyclization reactions in daffodil chromoplasts. Eur J Biochem. 1989 Sep 1;184(1):141–150. doi: 10.1111/j.1432-1033.1989.tb15000.x. [DOI] [PubMed] [Google Scholar]
- Bollivar D. W., Suzuki J. Y., Beatty J. T., Dobrowolski J. M., Bauer C. E. Directed mutational analysis of bacteriochlorophyll a biosynthesis in Rhodobacter capsulatus. J Mol Biol. 1994 Apr 15;237(5):622–640. doi: 10.1006/jmbi.1994.1260. [DOI] [PubMed] [Google Scholar]
- Bramley P. M., Mackenzie A. Regulation of carotenoid biosynthesis. Curr Top Cell Regul. 1988;29:291–343. doi: 10.1016/b978-0-12-152829-4.50009-4. [DOI] [PubMed] [Google Scholar]
- Carattoli A., Romano N., Ballario P., Morelli G., Macino G. The Neurospora crassa carotenoid biosynthetic gene (albino 3) reveals highly conserved regions among prenyltransferases. J Biol Chem. 1991 Mar 25;266(9):5854–5859. [PubMed] [Google Scholar]
- Chamovitz D., Misawa N., Sandmann G., Hirschberg J. Molecular cloning and expression in Escherichia coli of a cyanobacterial gene coding for phytoene synthase, a carotenoid biosynthesis enzyme. FEBS Lett. 1992 Jan 27;296(3):305–310. doi: 10.1016/0014-5793(92)80310-d. [DOI] [PubMed] [Google Scholar]
- Chamovitz D., Pecker I., Hirschberg J. The molecular basis of resistance to the herbicide norflurazon. Plant Mol Biol. 1991 Jun;16(6):967–974. doi: 10.1007/BF00016069. [DOI] [PubMed] [Google Scholar]
- Chamovitz D., Sandmann G., Hirschberg J. Molecular and biochemical characterization of herbicide-resistant mutants of cyanobacteria reveals that phytoene desaturation is a rate-limiting step in carotenoid biosynthesis. J Biol Chem. 1993 Aug 15;268(23):17348–17353. [PubMed] [Google Scholar]
- Chen A., Kroon P. A., Poulter C. D. Isoprenyl diphosphate synthases: protein sequence comparisons, a phylogenetic tree, and predictions of secondary structure. Protein Sci. 1994 Apr;3(4):600–607. doi: 10.1002/pro.5560030408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomber S. A., Chaudhri M., Connor A., Britton G., Hunter C. N. Localized transposon Tn5 mutagenesis of the photosynthetic gene cluster of Rhodobacter sphaeroides. Mol Microbiol. 1990 Jun;4(6):977–989. doi: 10.1111/j.1365-2958.1990.tb00670.x. [DOI] [PubMed] [Google Scholar]
- Cunningham F. X., Jr, Chamovitz D., Misawa N., Gantt E., Hirschberg J. Cloning and functional expression in Escherichia coli of a cyanobacterial gene for lycopene cyclase, the enzyme that catalyzes the biosynthesis of beta-carotene. FEBS Lett. 1993 Aug 9;328(1-2):130–138. doi: 10.1016/0014-5793(93)80980-9. [DOI] [PubMed] [Google Scholar]
- Deruère J., Römer S., d'Harlingue A., Backhaus R. A., Kuntz M., Camara B. Fibril assembly and carotenoid overaccumulation in chromoplasts: a model for supramolecular lipoprotein structures. Plant Cell. 1994 Jan;6(1):119–133. doi: 10.1105/tpc.6.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes M., Ruiz-Vázquez R., Murillo F. J. Growth phase dependence of the activation of a bacterial gene for carotenoid synthesis by blue light. EMBO J. 1993 Apr;12(4):1265–1275. doi: 10.1002/j.1460-2075.1993.tb05771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser P. D., Misawa N., Linden H., Yamano S., Kobayashi K., Sandmann G. Expression in Escherichia coli, purification, and reactivation of the recombinant Erwinia uredovora phytoene desaturase. J Biol Chem. 1992 Oct 5;267(28):19891–19895. [PubMed] [Google Scholar]
- GRIFFITHS M., SISTROM W. R., COHENBAZIRE G., STANIER R. Y., CALVIN M. Function of carotenoids in photosynthesis. Nature. 1955 Dec 24;176(4495):1211–1215. doi: 10.1038/1761211a0. [DOI] [PubMed] [Google Scholar]
- GRIFFITHS M., STANIER R. Y. Some mutational changes in the photosynthetic pigment system of Rhodopseudomonas spheroides. J Gen Microbiol. 1956 Jul;14(3):698–715. doi: 10.1099/00221287-14-3-698. [DOI] [PubMed] [Google Scholar]
- Garí E., Gibert I., Barbé J. Spontaneous and reversible high-frequency frameshifts originating a phase transition in the carotenoid biosynthesis pathway of the phototrophic bacterium Rhodobacter sphaeroides 2.4.1. Mol Gen Genet. 1992 Mar;232(1):74–80. doi: 10.1007/BF00299139. [DOI] [PubMed] [Google Scholar]
- Garí E., Toledo J. C., Gibert I., Barbé J. Nucleotide sequence of the methoxyneurosporene dehydrogenase gene from Rhodobacter sphaeroides: comparison with other bacterial carotenoid dehydrogenases. FEMS Microbiol Lett. 1992 May 15;72(1):103–108. doi: 10.1016/0378-1097(92)90497-c. [DOI] [PubMed] [Google Scholar]
- Giuliano G., Pollock D., Scolnik P. A. The gene crtI mediates the conversion of phytoene into colored carotenoids in Rhodopseudomonas capsulata. J Biol Chem. 1986 Oct 5;261(28):12925–12929. [PubMed] [Google Scholar]
- Hodgson D. A. Light-induced carotenogenesis in Myxococcus xanthus: genetic analysis of the carR region. Mol Microbiol. 1993 Feb;7(3):471–488. doi: 10.1111/j.1365-2958.1993.tb01138.x. [DOI] [PubMed] [Google Scholar]
- Hoshino T., Fujii R., Nakahara T. Molecular cloning and sequence analysis of the crtB gene of Thermus thermophilus HB27, an extreme thermophile producing carotenoid pigments. Appl Environ Microbiol. 1993 Sep;59(9):3150–3153. doi: 10.1128/aem.59.9.3150-3153.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugueney P., Römer S., Kuntz M., Camara B. Characterization and molecular cloning of a flavoprotein catalyzing the synthesis of phytofluene and zeta-carotene in Capsicum chromoplasts. Eur J Biochem. 1992 Oct 1;209(1):399–407. doi: 10.1111/j.1432-1033.1992.tb17302.x. [DOI] [PubMed] [Google Scholar]
- Hundle B. S., Beyer P., Kleinig H., Englert G., Hearst J. E. Carotenoids of Erwinia herbicola and an Escherichia coli HB101 strain carrying the Erwinia herbicola carotenoid gene cluster. Photochem Photobiol. 1991 Jul;54(1):89–93. doi: 10.1111/j.1751-1097.1991.tb01989.x. [DOI] [PubMed] [Google Scholar]
- Hundle B. S., O'Brien D. A., Alberti M., Beyer P., Hearst J. E. Functional expression of zeaxanthin glucosyltransferase from Erwinia herbicola and a proposed uridine diphosphate binding site. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9321–9325. doi: 10.1073/pnas.89.19.9321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundle B. S., O'Brien D. A., Beyer P., Kleinig H., Hearst J. E. In vitro expression and activity of lycopene cyclase and beta-carotene hydroxylase from Erwinia herbicola. FEBS Lett. 1993 Jan 11;315(3):329–334. doi: 10.1016/0014-5793(93)81188-6. [DOI] [PubMed] [Google Scholar]
- Hunter C. N., Hundle B. S., Hearst J. E., Lang H. P., Gardiner A. T., Takaichi S., Cogdell R. J. Introduction of new carotenoids into the bacterial photosynthetic apparatus by combining the carotenoid biosynthetic pathways of Erwinia herbicola and Rhodobacter sphaeroides. J Bacteriol. 1994 Jun;176(12):3692–3697. doi: 10.1128/jb.176.12.3692-3697.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinig H. On the utilization in vivo of lycopene and phytoene as precursors for the formation of carotenoid glucoside ester and on the regulation of carotenoid biosynthesis in Myxococcus fulvus. Eur J Biochem. 1975 Sep 1;57(1):301–308. doi: 10.1111/j.1432-1033.1975.tb02301.x. [DOI] [PubMed] [Google Scholar]
- Kuntz M., Römer S., Suire C., Hugueney P., Weil J. H., Schantz R., Camara B. Identification of a cDNA for the plastid-located geranylgeranyl pyrophosphate synthase from Capsicum annuum: correlative increase in enzyme activity and transcript level during fruit ripening. Plant J. 1992 Jan;2(1):25–34. doi: 10.1111/j.1365-313x.1992.00025.x. [DOI] [PubMed] [Google Scholar]
- Kühlbrandt W., Wang D. N., Fujiyoshi Y. Atomic model of plant light-harvesting complex by electron crystallography. Nature. 1994 Feb 17;367(6464):614–621. doi: 10.1038/367614a0. [DOI] [PubMed] [Google Scholar]
- Lee L. Y., Liu S. T. Characterization of the yellow-pigment genes of Erwinia herbicola. Mol Microbiol. 1991 Jan;5(1):217–224. doi: 10.1111/j.1365-2958.1991.tb01842.x. [DOI] [PubMed] [Google Scholar]
- Linden H., Misawa N., Saito T., Sandmann G. A novel carotenoid biosynthesis gene coding for zeta-carotene desaturase: functional expression, sequence and phylogenetic origin. Plant Mol Biol. 1994 Jan;24(2):369–379. doi: 10.1007/BF00020174. [DOI] [PubMed] [Google Scholar]
- Marrs B. Mobilization of the genes for photosynthesis from Rhodopseudomonas capsulata by a promiscuous plasmid. J Bacteriol. 1981 Jun;146(3):1003–1012. doi: 10.1128/jb.146.3.1003-1012.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Férez I. M., Vioque A. Nucleotide sequence of the phytoene desaturase gene from Synechocystis sp. PCC 6803 and characterization of a new mutation which confers resistance to the herbicide norflurazon. Plant Mol Biol. 1992 Mar;18(5):981–983. doi: 10.1007/BF00019213. [DOI] [PubMed] [Google Scholar]
- Martínez-Férez I., Fernández-González B., Sandmann G., Vioque A. Cloning and expression in Escherichia coli of the gene coding for phytoene synthase from the cyanobacterium Synechocystis sp. PCC6803. Biochim Biophys Acta. 1994 Jun 21;1218(2):145–152. doi: 10.1016/0167-4781(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Martínez-Laborda A., Balsalobre J. M., Fontes M., Murillo F. J. Accumulation of carotenoids in structural and regulatory mutants of the bacterium Myxococcus xanthus. Mol Gen Genet. 1990 Sep;223(2):205–210. doi: 10.1007/BF00265055. [DOI] [PubMed] [Google Scholar]
- Math S. K., Hearst J. E., Poulter C. D. The crtE gene in Erwinia herbicola encodes geranylgeranyl diphosphate synthase. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6761–6764. doi: 10.1073/pnas.89.15.6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan S. J., Gorham H. C., Hodgson D. A. Light-induced carotenogenesis in Myxococcus xanthus: DNA sequence analysis of the carR region. Mol Microbiol. 1993 Nov;10(4):713–735. doi: 10.1111/j.1365-2958.1993.tb00943.x. [DOI] [PubMed] [Google Scholar]
- Michalowski C. B., Löffelhardt W., Bohnert H. J. An ORF323 with homology to crtE, specifying prephytoene pyrophosphate dehydrogenase, is encoded by cyanelle DNA in the eukaryotic alga Cyanophora paradoxa. J Biol Chem. 1991 Jun 25;266(18):11866–11870. [PubMed] [Google Scholar]
- Misawa N., Nakagawa M., Kobayashi K., Yamano S., Izawa Y., Nakamura K., Harashima K. Elucidation of the Erwinia uredovora carotenoid biosynthetic pathway by functional analysis of gene products expressed in Escherichia coli. J Bacteriol. 1990 Dec;172(12):6704–6712. doi: 10.1128/jb.172.12.6704-6712.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misawa N., Yamano S., Ikenaga H. Production of beta-carotene in Zymomonas mobilis and Agrobacterium tumefaciens by introduction of the biosynthesis genes from Erwinia uredovora. Appl Environ Microbiol. 1991 Jun;57(6):1847–1849. doi: 10.1128/aem.57.6.1847-1849.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misawa N., Yamano S., Linden H., de Felipe M. R., Lucas M., Ikenaga H., Sandmann G. Functional expression of the Erwinia uredovora carotenoid biosynthesis gene crtl in transgenic plants showing an increase of beta-carotene biosynthesis activity and resistance to the bleaching herbicide norflurazon. Plant J. 1993 Nov;4(5):833–840. doi: 10.1046/j.1365-313x.1993.04050833.x. [DOI] [PubMed] [Google Scholar]
- Omer C. A., Gibbs J. B. Protein prenylation in eukaryotic microorganisms: genetics, biology and biochemistry. Mol Microbiol. 1994 Jan;11(2):219–225. doi: 10.1111/j.1365-2958.1994.tb00302.x. [DOI] [PubMed] [Google Scholar]
- Ourisson G., Rohmer M., Poralla K. Prokaryotic hopanoids and other polyterpenoid sterol surrogates. Annu Rev Microbiol. 1987;41:301–333. doi: 10.1146/annurev.mi.41.100187.001505. [DOI] [PubMed] [Google Scholar]
- Pecker I., Chamovitz D., Linden H., Sandmann G., Hirschberg J. A single polypeptide catalyzing the conversion of phytoene to zeta-carotene is transcriptionally regulated during tomato fruit ripening. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4962–4966. doi: 10.1073/pnas.89.11.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfold R. J., Pemberton J. M. Sequencing, chromosomal inactivation, and functional expression in Escherichia coli of ppsR, a gene which represses carotenoid and bacteriochlorophyll synthesis in Rhodobacter sphaeroides. J Bacteriol. 1994 May;176(10):2869–2876. doi: 10.1128/jb.176.10.2869-2876.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry K. L., Simonitch T. A., Harrison-Lavoie K. J., Liu S. T. Cloning and regulation of Erwinia herbicola pigment genes. J Bacteriol. 1986 Nov;168(2):607–612. doi: 10.1128/jb.168.2.607-612.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray J., Bird C., Maunders M., Grierson D., Schuch W. Sequence of pTOM5, a ripening related cDNA from tomato. Nucleic Acids Res. 1987 Dec 23;15(24):10587–10587. doi: 10.1093/nar/15.24.10587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray J., Moureau P., Bird C., Bird A., Grierson D., Maunders M., Truesdale M., Bramley P., Schuch W. Cloning and characterization of a gene involved in phytoene synthesis from tomato. Plant Mol Biol. 1992 Jun;19(3):401–404. doi: 10.1007/BF00023387. [DOI] [PubMed] [Google Scholar]
- Reith M., Munholland J. A High-Resolution Gene Map of the Chloroplast Genome of the Red Alga Porphyra purpurea. Plant Cell. 1993 Apr;5(4):465–475. doi: 10.1105/tpc.5.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock C. D., Zeevaart J. A. The aba mutant of Arabidopsis thaliana is impaired in epoxy-carotenoid biosynthesis. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7496–7499. doi: 10.1073/pnas.88.17.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Vázquez R., Fontes M., Murillo F. J. Clustering and co-ordinated activation of carotenoid genes in Myxococcus xanthus by blue light. Mol Microbiol. 1993 Oct;10(1):25–34. doi: 10.1111/j.1365-2958.1993.tb00900.x. [DOI] [PubMed] [Google Scholar]
- Römer S., Hugueney P., Bouvier F., Camara B., Kuntz M. Expression of the genes encoding the early carotenoid biosynthetic enzymes in Capsicum annuum. Biochem Biophys Res Commun. 1993 Nov 15;196(3):1414–1421. doi: 10.1006/bbrc.1993.2410. [DOI] [PubMed] [Google Scholar]
- Sandmann G., Misawa N. New functional assignment of the carotenogenic genes crtB and crtE with constructs of these genes from Erwinia species. FEMS Microbiol Lett. 1992 Jan 15;69(3):253–257. doi: 10.1016/0378-1097(92)90656-9. [DOI] [PubMed] [Google Scholar]
- Sandmann G., Misawa N., Wiedemann M., Vittorioso P., Carattoli A., Morelli G., Macino G. Functional identification of al-3 from Neurospora crassa as the gene for geranylgeranyl pyrophosphate synthase by complementation with crt genes, in vitro characterization of the gene product and mutant analysis. J Photochem Photobiol B. 1993 May;18(2-3):245–251. doi: 10.1016/1011-1344(93)80071-g. [DOI] [PubMed] [Google Scholar]
- Schmidhauser T. J., Lauter F. R., Russo V. E., Yanofsky C. Cloning, sequence, and photoregulation of al-1, a carotenoid biosynthetic gene of Neurospora crassa. Mol Cell Biol. 1990 Oct;10(10):5064–5070. doi: 10.1128/mcb.10.10.5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnik P. A., Walker M. A., Marrs B. L. Biosynthesis of carotenoids derived from neurosporene in Rhodopseudomonas capsulata. J Biol Chem. 1980 Mar 25;255(6):2427–2432. [PubMed] [Google Scholar]
- Sherman M. M., Petersen L. A., Poulter C. D. Isolation and characterization of isoprene mutants of Escherichia coli. J Bacteriol. 1989 Jul;171(7):3619–3628. doi: 10.1128/jb.171.7.3619-3628.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. P., Cohen S. N., Clark W. G., Marrs B. L. Alignment of genetic and restriction maps of the photosynthesis region of the Rhodopseudomonas capsulata chromosome by a conjugation-mediated marker rescue technique. J Bacteriol. 1983 May;154(2):580–590. doi: 10.1128/jb.154.2.580-590.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K. Y., Lai E. M., Lee L. Y., Lin T. P., Hung C. H., Chen C. L., Chang Y. S., Liu S. T. Analysis of the gene cluster encoding carotenoid biosynthesis in Erwinia herbicola Eho13. Microbiology. 1994 Feb;140(Pt 2):331–339. doi: 10.1099/13500872-140-2-331. [DOI] [PubMed] [Google Scholar]
- Tuveson R. W., Larson R. A., Kagan J. Role of cloned carotenoid genes expressed in Escherichia coli in protecting against inactivation by near-UV light and specific phototoxic molecules. J Bacteriol. 1988 Oct;170(10):4675–4680. doi: 10.1128/jb.170.10.4675-4680.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen H. C., Marrs B. Map of genes for carotenoid and bacteriochlorophyll biosynthesis in Rhodopseudomonas capsulata. J Bacteriol. 1976 May;126(2):619–629. doi: 10.1128/jb.126.2.619-629.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. A., Bauer C. E., Williams J. C., Marrs B. L. Genetic evidence for superoperonal organization of genes for photosynthetic pigments and pigment-binding proteins in Rhodobacter capsulatus. Mol Gen Genet. 1989 Jul;218(1):1–12. doi: 10.1007/BF00330558. [DOI] [PubMed] [Google Scholar]
- Zsebo K. M., Hearst J. E. Genetic-physical mapping of a photosynthetic gene cluster from R. capsulata. Cell. 1984 Jul;37(3):937–947. doi: 10.1016/0092-8674(84)90428-8. [DOI] [PubMed] [Google Scholar]


