Abstract
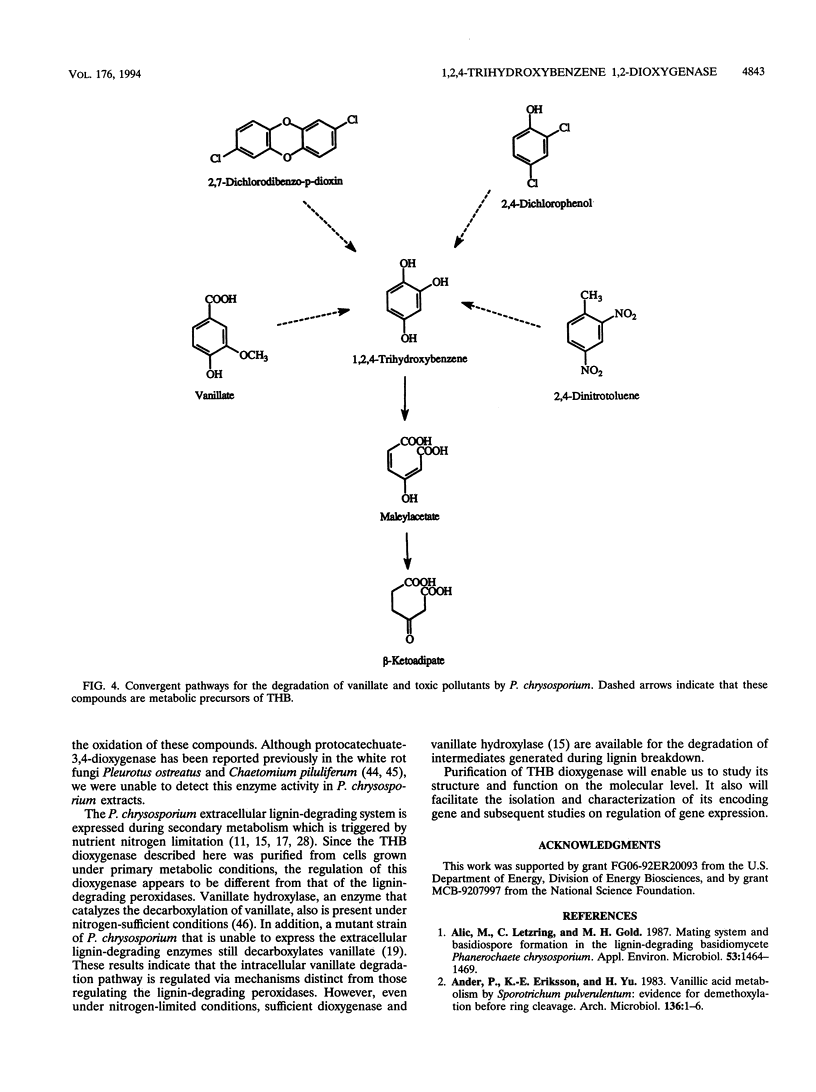
1,2,4-Trihydroxybenzene (THB) is an intermediate in the Phanerochaete chrysosporium degradation of vanillate and aromatic pollutants. A P. chrysosporium intracellular enzyme able to oxidatively cleave the aromatic ring of THB was purified by ammonium sulfate precipitation, hydrophobic and ion-exchange chromatographies, and native gel electrophoresis. The native protein has a molecular mass of 90 kDa and a subunit mass of 45 kDa. The enzyme catalyzes an intradiol cleavage of the substrate aromatic ring to produce maleylacetate. 18O2 incorporation studies demonstrate that molecular oxygen is a cosubstrate in the reaction. The enzyme exhibits high substrate specificity for THB; however, catechol cleavage occurs at approximately 20% of the optimal rate. THB dioxygenase catalyzes a key step in the degradation pathway of vanillate, an intermediate in lignin degradation. Maleylacetate, the product of THB cleavage, is reduced to beta-ketoadipate by an NADPH-requiring enzyme present in partially purified extracts.
Full text
PDF

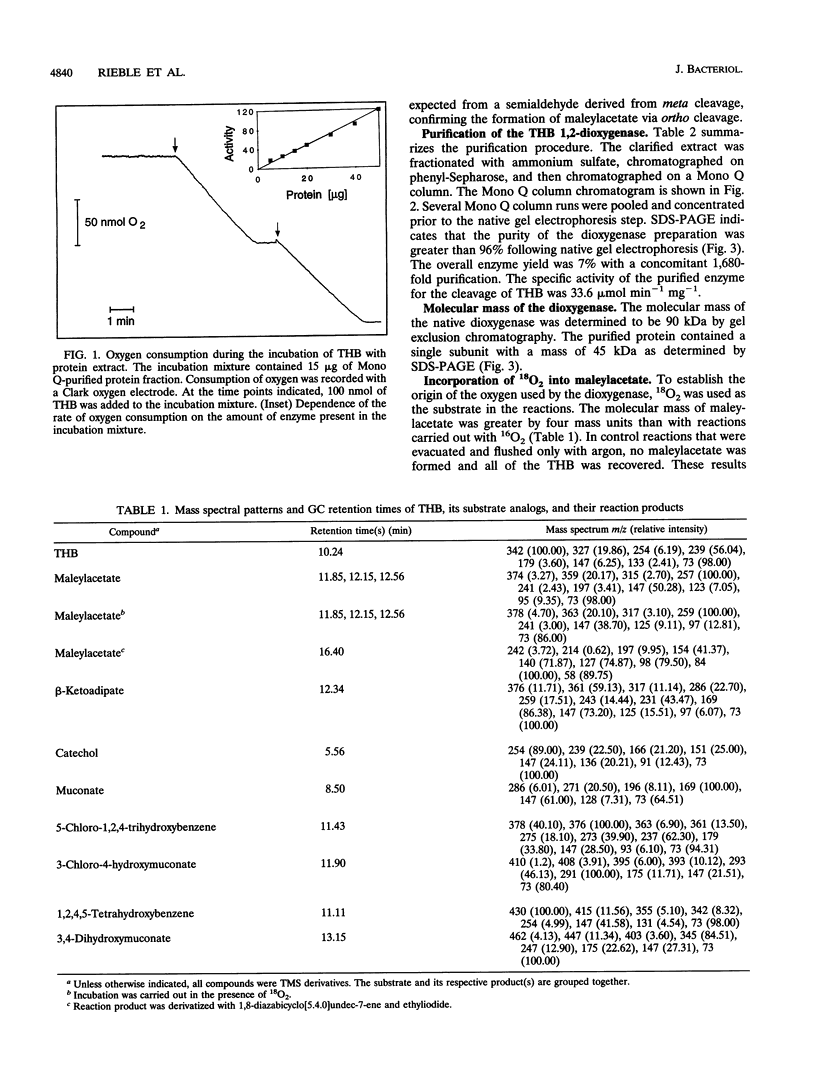
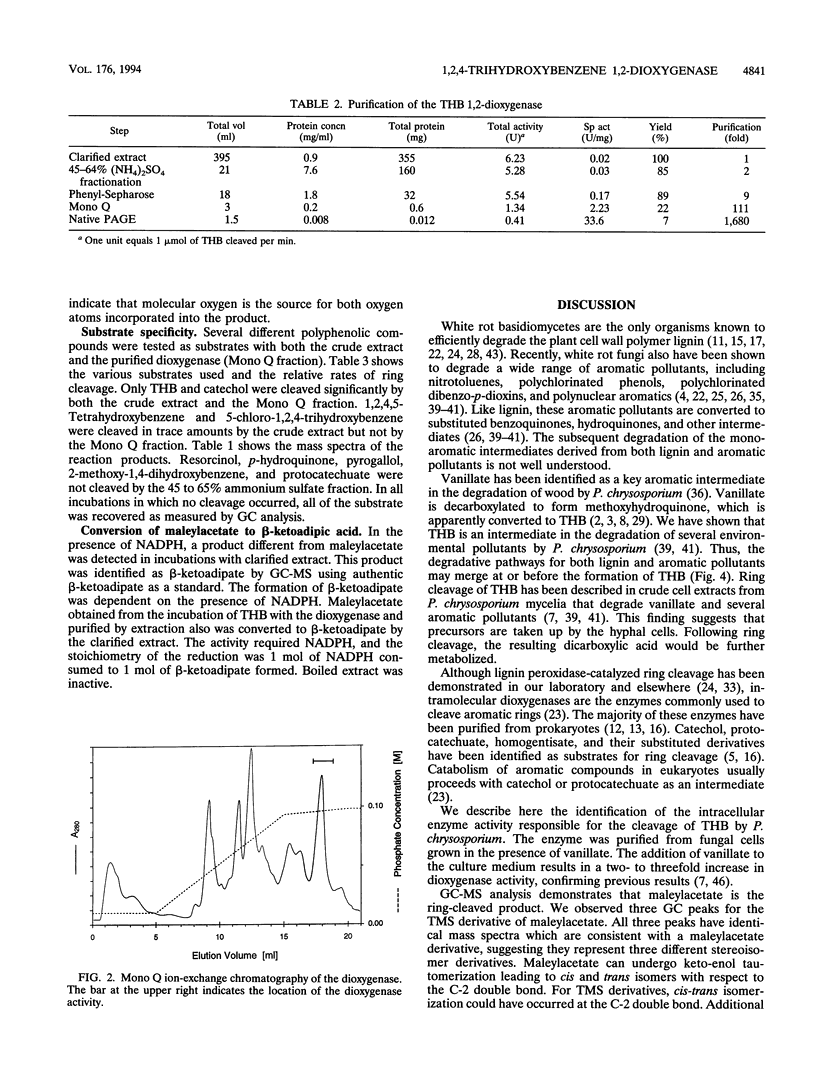
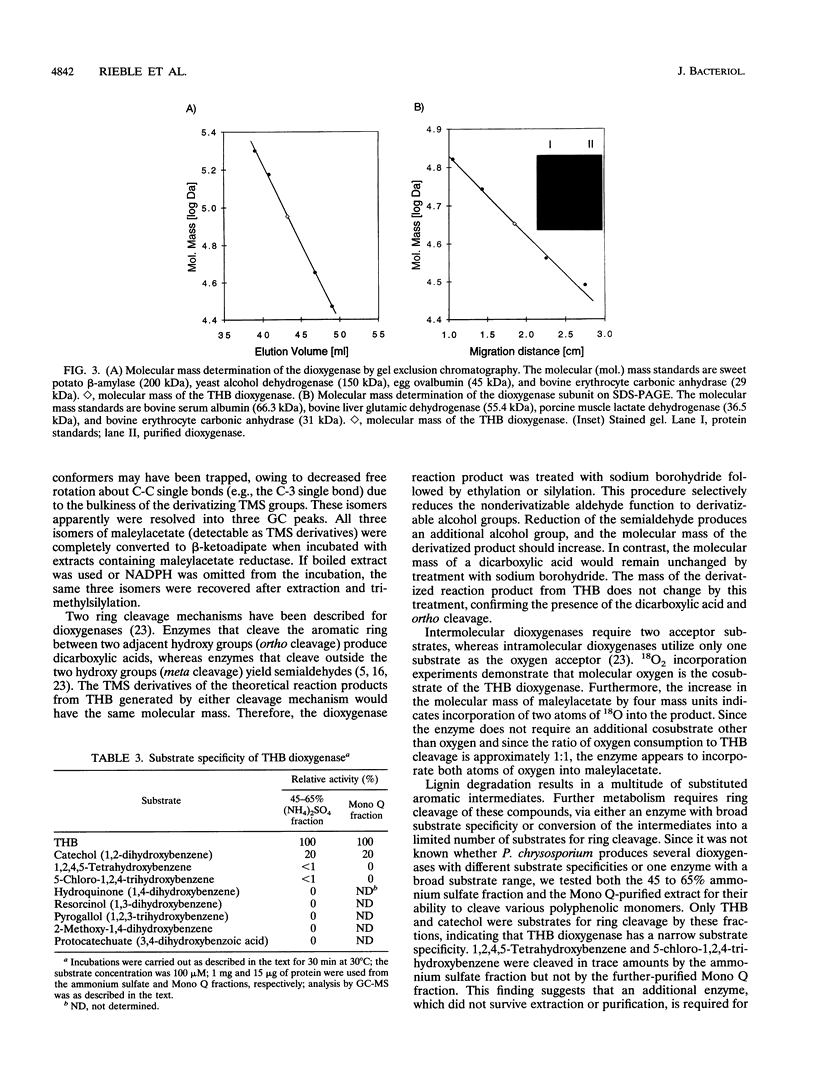

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alic M., Letzring C., Gold M. H. Mating System and Basidiospore Formation in the Lignin-Degrading Basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol. 1987 Jul;53(7):1464–1469. doi: 10.1128/aem.53.7.1464-1469.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buswell J. A., Hamp S., Eriksson K. E. Intracellular quinone reduction in Sporotrichum pulverulentum by a NAD(P)H:quinone oxidoreductase: possible role in vanillic acid catabolism. FEBS Lett. 1979 Dec 1;108(1):229–232. doi: 10.1016/0014-5793(79)81216-8. [DOI] [PubMed] [Google Scholar]
- Chapman P. J., Ribbons D. W. Metabolism of resorcinylic compounds by bacteria: alternative pathways for resorcinol catabolism in Pseudomonas putida. J Bacteriol. 1976 Mar;125(3):985–998. doi: 10.1128/jb.125.3.985-998.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman P. J., Ribbons D. W. Metabolism of resorcinylic compounds by bacteria: orcinol pathway in Pseudomonas putida. J Bacteriol. 1976 Mar;125(3):975–984. doi: 10.1128/jb.125.3.975-984.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Gold M. H., Alic M. Molecular biology of the lignin-degrading basidiomycete Phanerochaete chrysosporium. Microbiol Rev. 1993 Sep;57(3):605–622. doi: 10.1128/mr.57.3.605-622.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M. H., Kuwahara M., Chiu A. A., Glenn J. K. Purification and characterization of an extracellular H2O2-requiring diarylpropane oxygenase from the white rot basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys. 1984 Nov 1;234(2):353–362. doi: 10.1016/0003-9861(84)90280-7. [DOI] [PubMed] [Google Scholar]
- Joshi D. K., Gold M. H. Degradation of 2,4,5-trichlorophenol by the lignin-degrading basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol. 1993 Jun;59(6):1779–1785. doi: 10.1128/aem.59.6.1779-1785.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk T. K., Farrell R. L. Enzymatic "combustion": the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- Kirk T. K., Lorenz L. F. Methoxyhydroquinone, an intermediate of vanillate catabolism by Polyporus dichrous. Appl Microbiol. 1973 Aug;26(2):173–175. doi: 10.1128/am.26.2.173-175.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Spadaro J. T., Gold M. H., Renganathan V. Degradation of azo dyes by the lignin-degrading fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1992 Aug;58(8):2397–2401. doi: 10.1128/aem.58.8.2397-2401.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi L., Gupta A., Ki Paik W., Kim S. Purification and properties of protein methylase II from wheat germ. Eur J Biochem. 1982 Nov 15;128(2-3):349–354. doi: 10.1111/j.1432-1033.1982.tb06971.x. [DOI] [PubMed] [Google Scholar]
- Tuor U., Wariishi H., Schoemaker H. E., Gold M. H. Oxidation of phenolic arylglycerol beta-aryl ether lignin model compounds by manganese peroxidase from Phanerochaete chrysosporium: oxidative cleavage of an alpha-carbonyl model compound. Biochemistry. 1992 Jun 2;31(21):4986–4995. doi: 10.1021/bi00136a011. [DOI] [PubMed] [Google Scholar]
- Valli K., Brock B. J., Joshi D. K., Gold M. H. Degradation of 2,4-dinitrotoluene by the lignin-degrading fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1992 Jan;58(1):221–228. doi: 10.1128/aem.58.1.221-228.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valli K., Gold M. H. Degradation of 2,4-dichlorophenol by the lignin-degrading fungus Phanerochaete chrysosporium. J Bacteriol. 1991 Jan;173(1):345–352. doi: 10.1128/jb.173.1.345-352.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valli K., Wariishi H., Gold M. H. Degradation of 2,7-dichlorodibenzo-p-dioxin by the lignin-degrading basidiomycete Phanerochaete chrysosporium. J Bacteriol. 1992 Apr;174(7):2131–2137. doi: 10.1128/jb.174.7.2131-2137.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wariishi H., Valli K., Gold M. H. In vitro depolymerization of lignin by manganese peroxidase of Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1991 Apr 15;176(1):269–275. doi: 10.1016/0006-291x(91)90919-x. [DOI] [PubMed] [Google Scholar]
- Wojtaś-Wasilewska M., Trojanowski J., Luterek J. Aromatic ring cleavage of protocatechuic acid by the white-rot fungus Pleurotus ostreatus. Acta Biochim Pol. 1983;30(3-4):291–302. [PubMed] [Google Scholar]
- Wojtaś-Wasilewska M., Trojanowski J. Purification and properties of protocatechuate 3,4-dioxygenase from Chaetomium piluliferum induced with p-hydroxybenzoic acid. Acta Biochim Pol. 1980;27(1):21–34. [PubMed] [Google Scholar]