Abstract
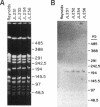
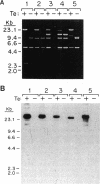
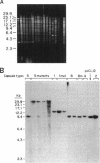
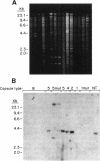
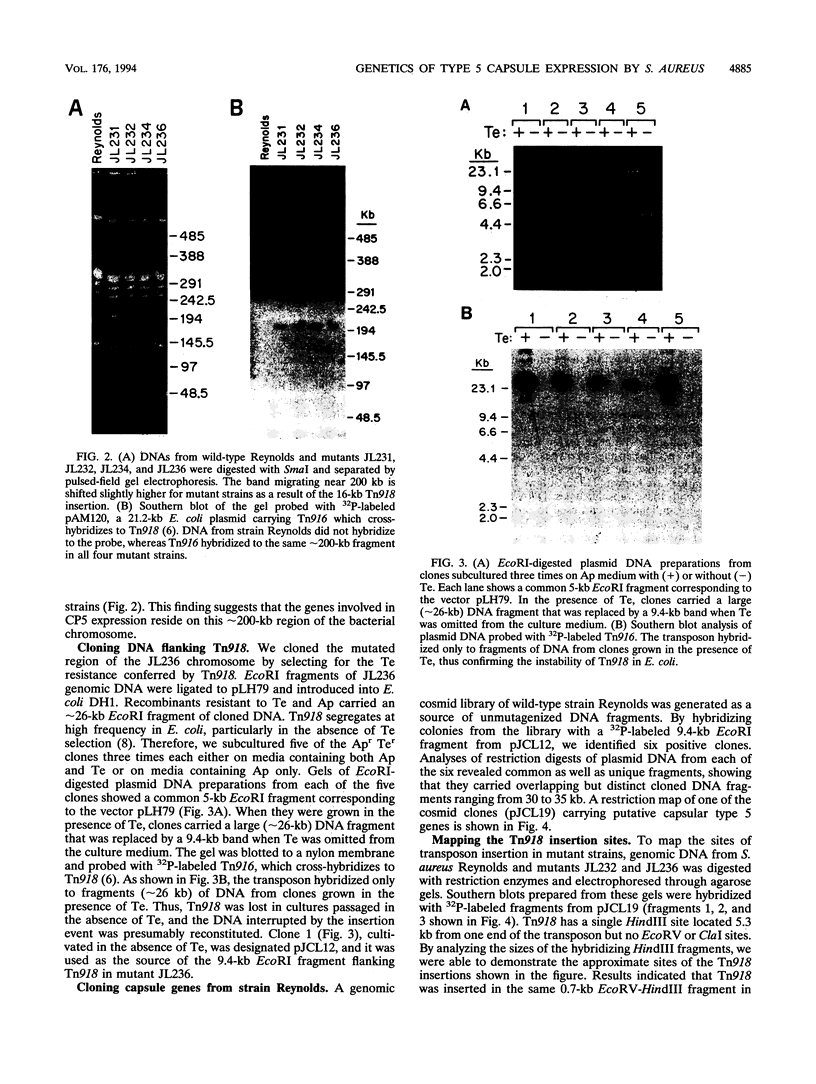
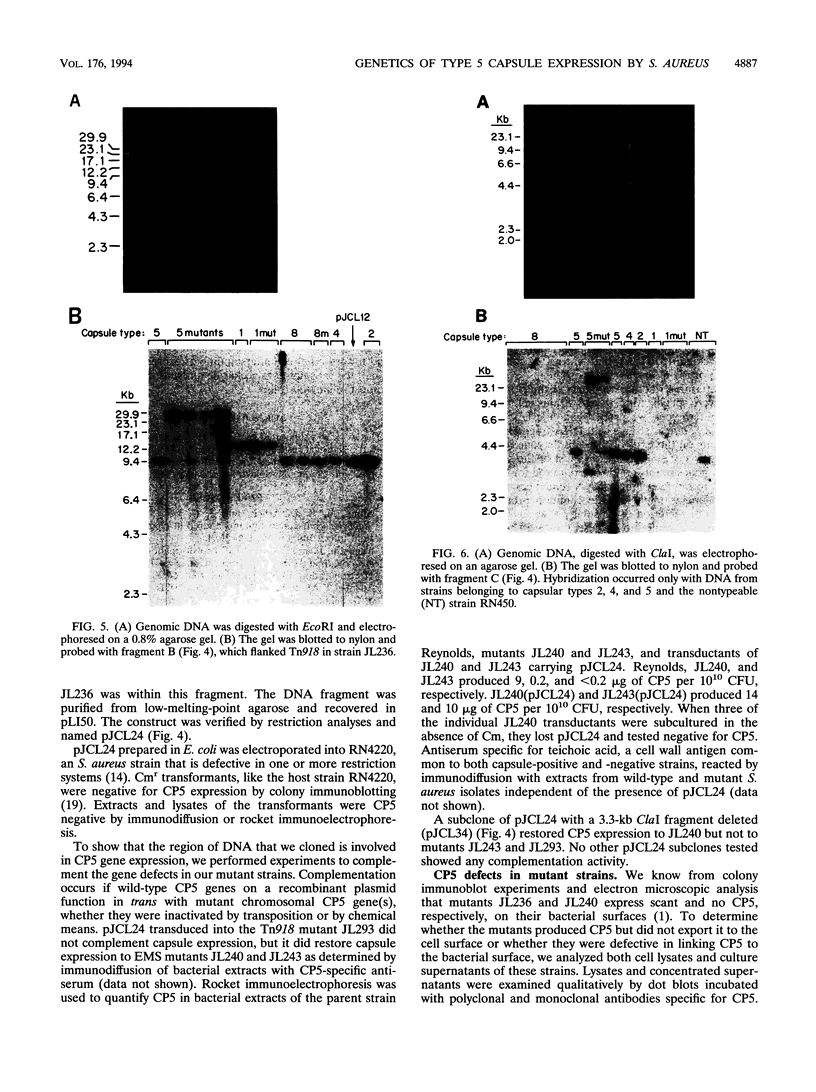
Capsules are produced by over 90% of Staphylococcus aureus strains, and approximately 25% of clinical isolates express type 5 capsular polysaccharide (CP5). We mutagenized the type 5 strain Reynolds with Tn918 to target genes involved in CP5 expression. From a capsule-deficient mutant, we cloned into a cosmid vector an approximately 26-kb EcoRI fragment containing the transposon insertion. In the absence of tetracycline selection, Tn918 was spontaneously excised, thereby resulting in a plasmid containing 9.4 kb of S. aureus DNA flanking the Tn918 insertion site. The 9.4-kb DNA fragment was used to screen a cosmid library prepared from the wild-type strain. Positive colonies were identified by colony hybridization, and a restriction map of one clone (pJCL19 with an approximately 34-kb insert) carrying the putative capsule gene region was constructed. Fragments of pJCL19 were used to probe genomic DNA digests from S. aureus strains of different capsular serotypes. Fragments on the ends of the cloned DNA hybridized to fragments of similar sizes in most of the strains examined. Blots hybridized to two fragments flanking the central region of the cloned DNA showed restriction fragment length polymorphism. A centrally located DNA fragment hybridized only to DNA from capsular types 2, 4, and 5. DNA from pJCL19 was subcloned to a shuttle vector for complementation studies. A 6.2-kb EcoRI-ClaI fragment complemented CP5 expression in a capsule-negative mutant derived by mutagenesis with ethyl methanesulfonate. These experiments provide the necessary groundwork for identifying genes involved in CP5 expression by S. aureus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albus A., Arbeit R. D., Lee J. C. Virulence of Staphylococcus aureus mutants altered in type 5 capsule production. Infect Immun. 1991 Mar;59(3):1008–1014. doi: 10.1128/iai.59.3.1008-1014.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin J., Götz F. Transformation of Staphylococcus epidermidis and other staphylococcal species with plasmid DNA by electroporation. FEMS Microbiol Lett. 1990 Jan 1;54(1-3):203–207. doi: 10.1016/0378-1097(90)90283-v. [DOI] [PubMed] [Google Scholar]
- Baddour L. M., Lowrance C., Albus A., Lowrance J. H., Anderson S. K., Lee J. C. Staphylococcus aureus microcapsule expression attenuates bacterial virulence in a rat model of experimental endocarditis. J Infect Dis. 1992 Apr;165(4):749–753. doi: 10.1093/infdis/165.4.749. [DOI] [PubMed] [Google Scholar]
- COHN Z. A. Determinants of infection in the peritoneal cavity. I. Response to and fate of Staphylococcus aureus and Staphylococcus albus in the mouse. Yale J Biol Med. 1962 Aug;35:12–28. [PMC free article] [PubMed] [Google Scholar]
- Chitnis C. E., Ohman D. E. Genetic analysis of the alginate biosynthetic gene cluster of Pseudomonas aeruginosa shows evidence of an operonic structure. Mol Microbiol. 1993 May;8(3):583–593. doi: 10.1111/j.1365-2958.1993.tb01602.x. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., An F. Y., White B. A., Gawron-Burke C. Streptococcus faecalis sex pheromone (cAM373) also produced by Staphylococcus aureus and identification of a conjugative transposon (Tn918). J Bacteriol. 1985 Jun;162(3):1212–1220. doi: 10.1128/jb.162.3.1212-1220.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawron-Burke C., Clewell D. B. Regeneration of insertionally inactivated streptococcal DNA fragments after excision of transposon Tn916 in Escherichia coli: strategy for targeting and cloning of genes from gram-positive bacteria. J Bacteriol. 1984 Jul;159(1):214–221. doi: 10.1128/jb.159.1.214-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert I. Dissociation in an Encapsulated Staphylococcus. J Bacteriol. 1931 Mar;21(3):157–160. doi: 10.1128/jb.21.3.157-160.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Kasatiya S. S., Baldwin J. N. Nature of the determinant of tetracycline resistance in Staphylococcus aureus. Can J Microbiol. 1967 Aug;13(8):1079–1086. doi: 10.1139/m67-144. [DOI] [PubMed] [Google Scholar]
- Koenig M. G., Melly M. A. The importance of surface antigens in staphylococcal virulence. Ann N Y Acad Sci. 1965 Jul 23;128(1):231–250. doi: 10.1111/j.1749-6632.1965.tb11641.x. [DOI] [PubMed] [Google Scholar]
- Kreiswirth B. N., Löfdahl S., Betley M. J., O'Reilly M., Schlievert P. M., Bergdoll M. S., Novick R. P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983 Oct 20;305(5936):709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- Kroll J. S., Zamze S., Loynds B., Moxon E. R. Common organization of chromosomal loci for production of different capsular polysaccharides in Haemophilus influenzae. J Bacteriol. 1989 Jun;171(6):3343–3347. doi: 10.1128/jb.171.6.3343-3347.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers J. M., Heggen L. M., Rubens C. E. Molecular analysis of a region of the group B streptococcus chromosome involved in type III capsule expression. Infect Immun. 1989 Oct;57(10):3058–3065. doi: 10.1128/iai.57.10.3058-3065.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Y., Buranen S. L., Ye Z. H. Construction of single-copy integration vectors for Staphylococcus aureus. Gene. 1991 Jul 15;103(1):101–105. doi: 10.1016/0378-1119(91)90399-v. [DOI] [PubMed] [Google Scholar]
- Lee C. Y. Cloning of genes affecting capsule expression in Staphylococcus aureus strain M. Mol Microbiol. 1992 Jun;6(11):1515–1522. doi: 10.1111/j.1365-2958.1992.tb00872.x. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Liu M. J., Parsonnet J., Arbeit R. D. Expression of type 8 capsular polysaccharide and production of toxic shock syndrome toxin 1 are associated among vaginal isolates of Staphylococcus aureus. J Clin Microbiol. 1990 Dec;28(12):2612–2615. doi: 10.1128/jcm.28.12.2612-2615.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., Takeda S., Livolsi P. J., Paoletti L. C. Effects of in vitro and in vivo growth conditions on expression of type 8 capsular polysaccharide by Staphylococcus aureus. Infect Immun. 1993 May;61(5):1853–1858. doi: 10.1128/iai.61.5.1853-1858.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melly M. A., Duke L. J., Liau D. F., Hash J. H. Biological properties of the encapsulated Staphylococcus aureus M. Infect Immun. 1974 Aug;10(2):389–397. doi: 10.1128/iai.10.2.389-397.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau M., Richards J. C., Fournier J. M., Byrd R. A., Karakawa W. W., Vann W. F. Structure of the type 5 capsular polysaccharide of Staphylococcus aureus. Carbohydr Res. 1990 Jul 1;201(2):285–297. doi: 10.1016/0008-6215(90)84244-o. [DOI] [PubMed] [Google Scholar]
- Ohtomo T., Yoshida K. Encapsulation by transformation of strains of Staphylococcus aureus determined by the serum-soft agar technique. Zentralbl Bakteriol Orig A. 1978 Dec;242(4):436–445. [PubMed] [Google Scholar]
- Peng H. L., Novick R. P., Kreiswirth B., Kornblum J., Schlievert P. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J Bacteriol. 1988 Sep;170(9):4365–4372. doi: 10.1128/jb.170.9.4365-4372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts I. S., Mountford R., Hodge R., Jann K. B., Boulnois G. J. Common organization of gene clusters for production of different capsular polysaccharides (K antigens) in Escherichia coli. J Bacteriol. 1988 Mar;170(3):1305–1310. doi: 10.1128/jb.170.3.1305-1310.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. M., Parisi J. T., Vidal L., Baldwin J. N. Nature of the genetic determinant controlling encapsulation in Staphylococcus aureus Smith. Infect Immun. 1977 Jul;17(1):231–234. doi: 10.1128/iai.17.1.231-234.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompolinsky D., Samra Z., Karakawa W. W., Vann W. F., Schneerson R., Malik Z. Encapsulation and capsular types in isolates of Staphylococcus aureus from different sources and relationship to phage types. J Clin Microbiol. 1985 Nov;22(5):828–834. doi: 10.1128/jcm.22.5.828-834.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy F. A., 2nd Polysialylation: from bacteria to brains. Glycobiology. 1992 Feb;2(1):5–23. doi: 10.1093/glycob/2.1.5. [DOI] [PubMed] [Google Scholar]
- WILKINSON J. F. The extracellualr polysaccharides of bacteria. Bacteriol Rev. 1958 Mar;22(1):46–73. doi: 10.1128/br.22.1.46-73.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Arbeit R. D., Lee J. C. Phagocytic killing of encapsulated and microencapsulated Staphylococcus aureus by human polymorphonuclear leukocytes. Infect Immun. 1992 Apr;60(4):1358–1362. doi: 10.1128/iai.60.4.1358-1362.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]