Abstract
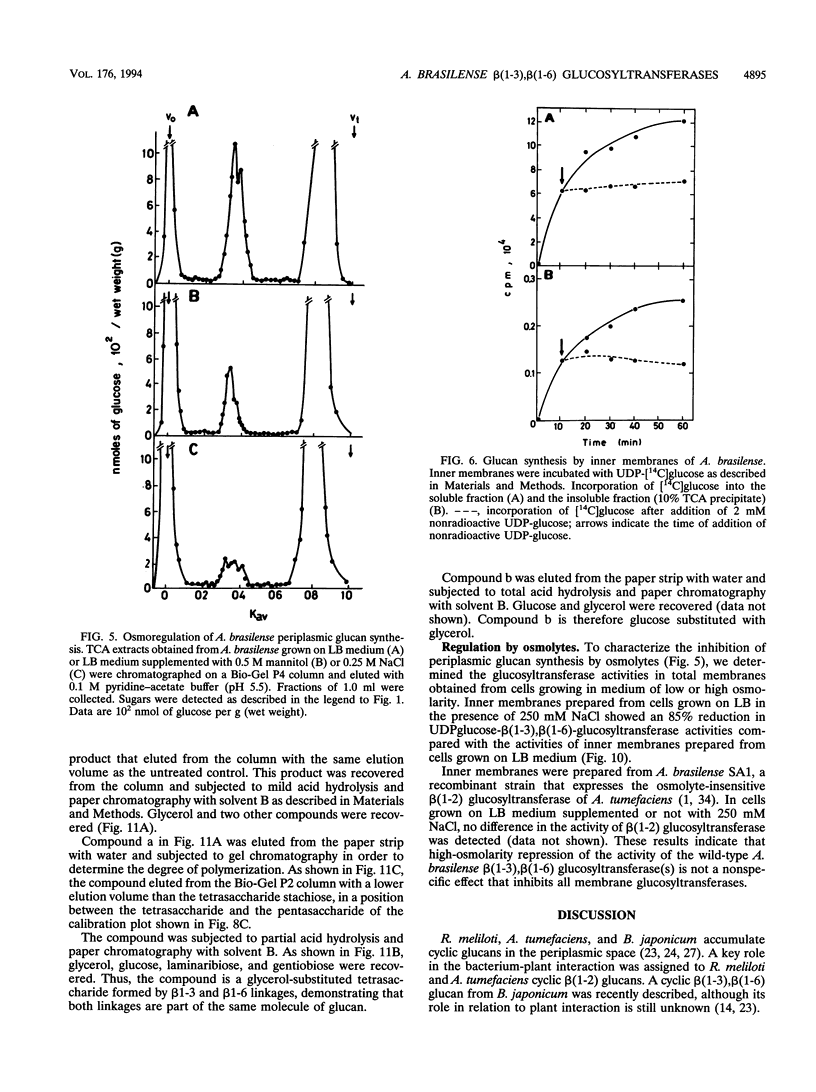
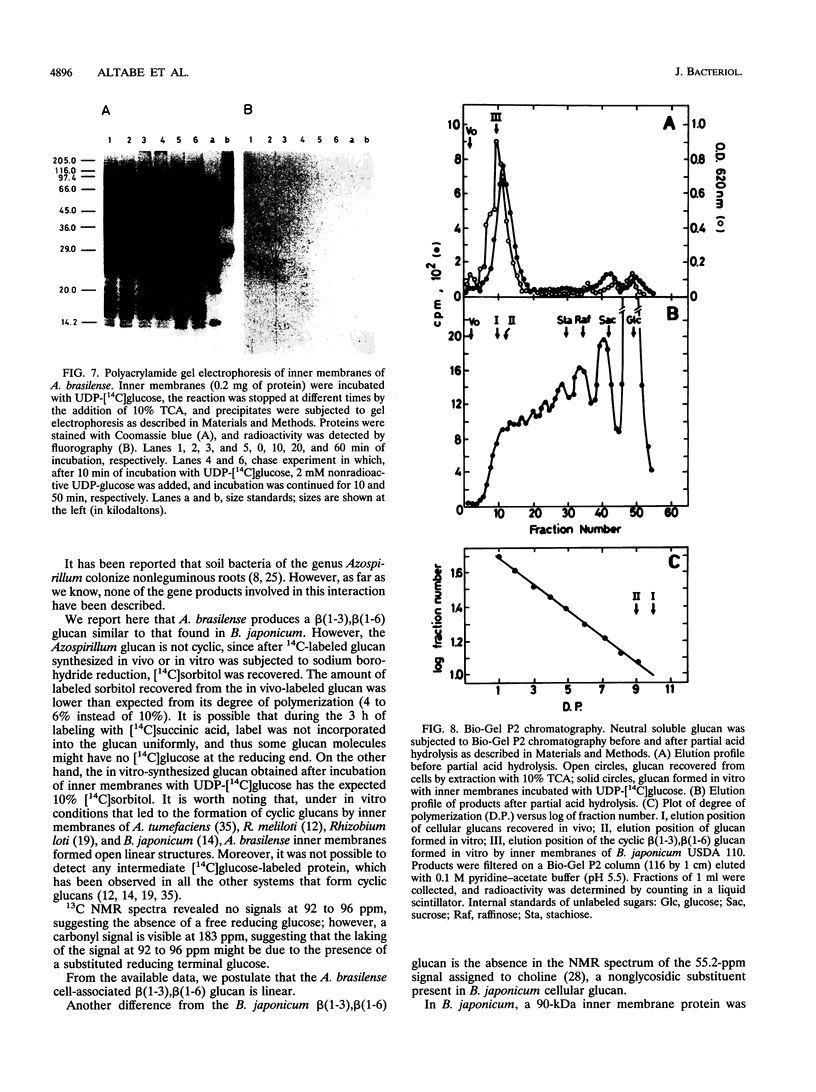
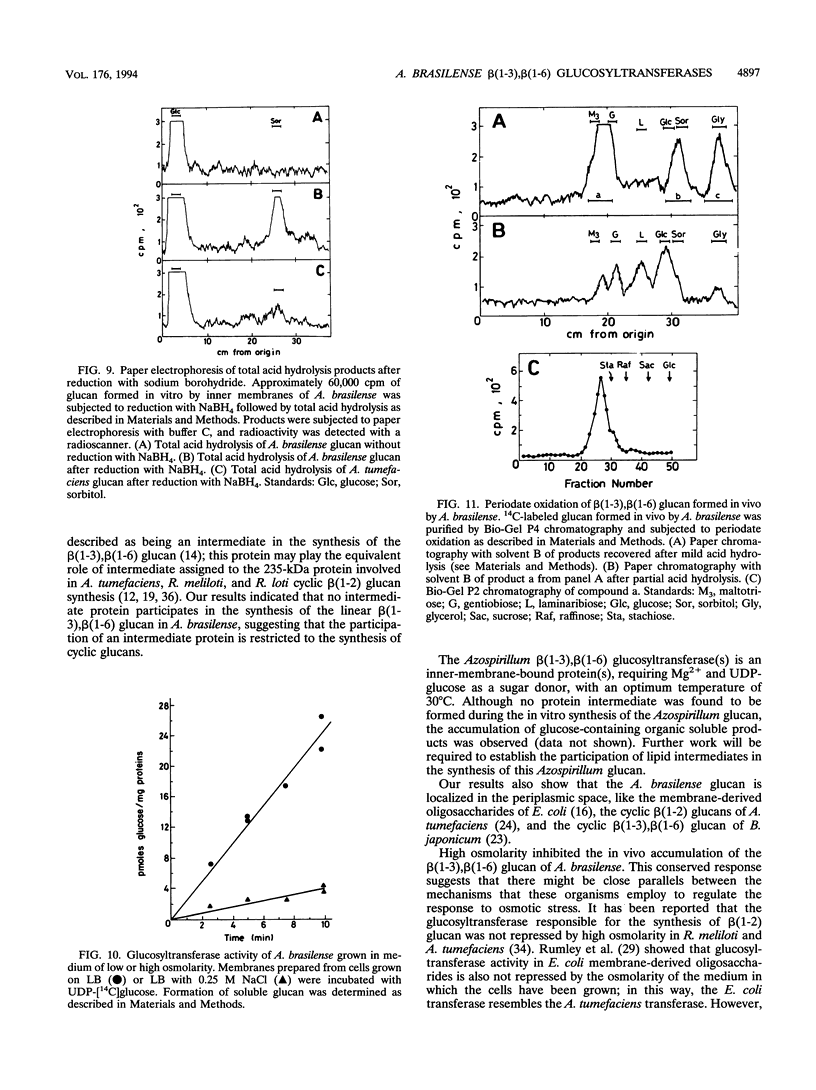
A linear beta(1-3),beta(1-6) glucan was detected in the periplasm of Azospirillum brasilense cells growing in a medium of low osmotic strength. This glucan was produced in vitro by purified bacterial inner membranes with UDP-glucose as the sugar donor in the presence of Mg2+. Growth in a high-osmotic-strength medium strongly reduced the amount of this glucan accumulated in the periplasmic space, and the inhibition was associated with a reduction in the enzymatic activity of the beta(1-3),beta(1-6) glucosyltransferase(s).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altabe S., Iñn de Iannino N., de Mendoza D., Ugalde R. A. Expression of the Agrobacterium tumefaciens chvB virulence region in Azospirillum spp. J Bacteriol. 1990 May;172(5):2563–2567. doi: 10.1128/jb.172.5.2563-2567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann M., Simon H., Schneider K. H., Danneel H. J., Küster U., Giffhorn F. Susceptibility of Rhodobacter sphaeroides to beta-lactam antibiotics: isolation and characterization of a periplasmic beta-lactamase (cephalosporinase). J Bacteriol. 1989 Jan;171(1):308–313. doi: 10.1128/jb.171.1.308-313.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. L., Miller K. J. A novel membrane-bound glucosyltransferase from Bradyrhizobium japonicum. J Bacteriol. 1991 Jul;173(14):4271–4276. doi: 10.1128/jb.173.14.4271-4276.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtright J. B., Henning U. Malate dehydrogenase mutants in Escherichia coli K-12. J Bacteriol. 1970 Jun;102(3):722–728. doi: 10.1128/jb.102.3.722-728.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dylan T., Ielpi L., Stanfield S., Kashyap L., Douglas C., Yanofsky M., Nester E., Helinski D. R., Ditta G. Rhizobium meliloti genes required for nodule development are related to chromosomal virulence genes in Agrobacterium tumefaciens. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4403–4407. doi: 10.1073/pnas.83.12.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOSTER A. B. Zone electrophoresis of carbohydrates. Adv Carbohydr Chem. 1957;12:81–115. doi: 10.1016/s0096-5332(08)60205-2. [DOI] [PubMed] [Google Scholar]
- Geremia R. A., Cavaignac S., Zorreguieta A., Toro N., Olivares J., Ugalde R. A. A Rhizobium meliloti mutant that forms ineffective pseudonodules in alfalfa produces exopolysaccharide but fails to form beta-(1----2) glucan. J Bacteriol. 1987 Feb;169(2):880–884. doi: 10.1128/jb.169.2.880-884.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy E. P. Osmotic regulation and the biosynthesis of membrane-derived oligosaccharides in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1092–1095. doi: 10.1073/pnas.79.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix J. M., Loubens I., Tempête M., Menichi B., Bohin J. P. The mdoA locus of Escherichia coli consists of an operon under osmotic control. Mol Microbiol. 1991 Jul;5(7):1745–1753. doi: 10.1111/j.1365-2958.1991.tb01924.x. [DOI] [PubMed] [Google Scholar]
- Leigh J. A., Signer E. R., Walker G. C. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6231–6235. doi: 10.1073/pnas.82.18.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S., Reed J. W., Himawan J., Walker G. C. Genetic analysis of a cluster of genes required for synthesis of the calcofluor-binding exopolysaccharide of Rhizobium meliloti. J Bacteriol. 1988 Sep;170(9):4239–4248. doi: 10.1128/jb.170.9.4239-4248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. J., Gore R. S., Johnson R., Benesi A. J., Reinhold V. N. Cell-associated oligosaccharides of Bradyrhizobium spp. J Bacteriol. 1990 Jan;172(1):136–142. doi: 10.1128/jb.172.1.136-142.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. J., Kennedy E. P., Reinhold V. N. Osmotic adaptation by gram-negative bacteria: possible role for periplasmic oligosaccharides. Science. 1986 Jan 3;231(4733):48–51. doi: 10.1126/science.3941890. [DOI] [PubMed] [Google Scholar]
- Puvanesarajah V., Schell F. M., Stacey G., Douglas C. J., Nester E. W. Role for 2-linked-beta-D-glucan in the virulence of Agrobacterium tumefaciens. J Bacteriol. 1985 Oct;164(1):102–106. doi: 10.1128/jb.164.1.102-106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolin D. B., Pfeffer P. E., Osman S. F., Szwergold B. S., Kappler F., Benesi A. J. Structural studies of a phosphocholine substituted beta-(1,3);(1,6) macrocyclic glucan from Bradyrhizobium japonicum USDA 110. Biochim Biophys Acta. 1992 Jun 12;1116(3):215–225. doi: 10.1016/0304-4165(92)90014-l. [DOI] [PubMed] [Google Scholar]
- Rumley M. K., Therisod H., Weissborn A. C., Kennedy E. P. Mechanisms of regulation of the biosynthesis of membrane-derived oligosaccharides in Escherichia coli. J Biol Chem. 1992 Jun 15;267(17):11806–11810. [PubMed] [Google Scholar]
- Stanfield S. W., Ielpi L., O'Brochta D., Helinski D. R., Ditta G. S. The ndvA gene product of Rhizobium meliloti is required for beta-(1----2)glucan production and has homology to the ATP-binding export protein HlyB. J Bacteriol. 1988 Aug;170(8):3523–3530. doi: 10.1128/jb.170.8.3523-3530.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Tolmasky M. E., Staneloni R. J., Ugalde R. A., Leloir L. F. Lipid-bound sugars in Rhizobium meliloti. Arch Biochem Biophys. 1980 Aug;203(1):358–364. doi: 10.1016/0003-9861(80)90187-3. [DOI] [PubMed] [Google Scholar]
- Tung K. K., Nordin J. H. Structure of the tetrasaccharide produced by the hydrolysis of nigeran by the enzyme mycodextranase. Biochim Biophys Acta. 1968 Apr 16;158(1):154–156. doi: 10.1016/0304-4165(68)90084-6. [DOI] [PubMed] [Google Scholar]
- Zorreguieta A., Cavaignac S., Geremia R. A., Ugalde R. A. Osmotic regulation of beta(1-2) glucan synthesis in members of the family Rhizobiaceae. J Bacteriol. 1990 Aug;172(8):4701–4704. doi: 10.1128/jb.172.8.4701-4704.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorreguieta A., Geremia R. A., Cavaignac S., Cangelosi G. A., Nester E. W., Ugalde R. A. Identification of the product of an Agrobacterium tumefaciens chromosomal virulence gene. Mol Plant Microbe Interact. 1988 Mar;1(3):121–127. doi: 10.1094/mpmi-1-121. [DOI] [PubMed] [Google Scholar]
- Zorreguieta A., Ugalde R. A. Formation in Rhizobium and Agrobacterium spp. of a 235-kilodalton protein intermediate in beta-D(1-2) glucan synthesis. J Bacteriol. 1986 Sep;167(3):947–951. doi: 10.1128/jb.167.3.947-951.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Iannino N. I., Ugalde R. A. Biochemical characterization of avirulent Agrobacterium tumefaciens chvA mutants: synthesis and excretion of beta-(1-2)glucan. J Bacteriol. 1989 May;171(5):2842–2849. doi: 10.1128/jb.171.5.2842-2849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Iannino N. I., Ugalde R. A. Biosynthesis of cyclic beta-(1-3),beta-(1-6) glucan in Bradyrhizobium spp. Arch Microbiol. 1993;159(1):30–38. doi: 10.1007/BF00244260. [DOI] [PubMed] [Google Scholar]