Abstract
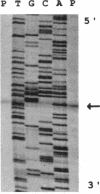
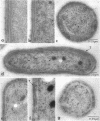
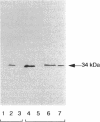
Growth of Synechococcus sp. strain PCC 7942 in iron-deficient media leads to the accumulation of an approximately 34-kDa protein. The gene encoding this protein, mapA (membrane-associated protein A), has been cloned and sequenced (GenBank accession number, L01621). The mapA transcript is not detectable in normally grown cultures but is stably accumulated by cells grown in iron-deficient media. However, the promoter sequence for this gene does not resemble other bacterial iron-regulated promoters described to date. The carboxyl-terminal region of the derived amino acid sequence of MapA resembles bacterial proteins involved in iron acquisition, whereas the amino-terminal end of MapA has a high degree of amino acid identity with the abundant, chloroplast envelope protein E37. An approach employing improved cellular fractionation techniques as well as electron microscopy and immunocytochemistry was essential in localizing MapA protein to the cytoplasmic membrane of Synechococcus sp. strain PCC 7942. When these cells were grown under iron-deficient conditions, a significant fraction of MapA could also be localized to the thylakoid membranes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angerer A., Gaisser S., Braun V. Nucleotide sequences of the sfuA, sfuB, and sfuC genes of Serratia marcescens suggest a periplasmic-binding-protein-dependent iron transport mechanism. J Bacteriol. 1990 Feb;172(2):572–578. doi: 10.1128/jb.172.2.572-578.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagg A., Neilands J. B. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol Rev. 1987 Dec;51(4):509–518. doi: 10.1128/mr.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berish S. A., Mietzner T. A., Mayer L. W., Genco C. A., Holloway B. P., Morse S. A. Molecular cloning and characterization of the structural gene for the major iron-regulated protein expressed by Neisseria gonorrhoeae. J Exp Med. 1990 May 1;171(5):1535–1546. doi: 10.1084/jem.171.5.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block M. A., Joyard J., Douce R. Purification and characterization of E37, a major chloroplast envelope protein. FEBS Lett. 1991 Aug 5;287(1-2):167–170. doi: 10.1016/0014-5793(91)80042-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bricker T. M., Sherman L. A. Triton X-114 phase fractionation of membrane proteins of the cyanobacterium Anacystis nidulans R2. Arch Biochem Biophys. 1984 Nov 15;235(1):204–211. doi: 10.1016/0003-9861(84)90269-8. [DOI] [PubMed] [Google Scholar]
- Crosa J. H. Genetics and molecular biology of siderophore-mediated iron transport in bacteria. Microbiol Rev. 1989 Dec;53(4):517–530. doi: 10.1128/mr.53.4.517-530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delepelaire P., Chua N. H. Lithium dodecyl sulfate/polyacrylamide gel electrophoresis of thylakoid membranes at 4 degrees C: Characterizations of two additional chlorophyll a-protein complexes. Proc Natl Acad Sci U S A. 1979 Jan;76(1):111–115. doi: 10.1073/pnas.76.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreses-Werringloer U., Fischer K., Wachter E., Link T. A., Flügge U. I. cDNA sequence and deduced amino acid sequence of the precursor of the 37-kDa inner envelope membrane polypeptide from spinach chloroplasts. Its transit peptide contains an amphiphilic alpha-helix as the only detectable structural element. Eur J Biochem. 1991 Jan 30;195(2):361–368. doi: 10.1111/j.1432-1033.1991.tb15714.x. [DOI] [PubMed] [Google Scholar]
- Elhai J., Wolk C. P. A versatile class of positive-selection vectors based on the nonviability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene. 1988 Aug 15;68(1):119–138. doi: 10.1016/0378-1119(88)90605-1. [DOI] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Guikema J. A., Sherman L. A. Influence of Iron Deprivation on the Membrane Composition of Anacystis nidulans. Plant Physiol. 1984 Jan;74(1):90–95. doi: 10.1104/pp.74.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness R. E., Chong P., Klein M. H. Identification of two iron-repressed periplasmic proteins in Haemophilus influenzae. J Bacteriol. 1992 Apr;174(8):2425–2430. doi: 10.1128/jb.174.8.2425-2430.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laudenbach D. E., Reith M. E., Straus N. A. Isolation, sequence analysis, and transcriptional studies of the flavodoxin gene from Anacystis nidulans R2. J Bacteriol. 1988 Jan;170(1):258–265. doi: 10.1128/jb.170.1.258-265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Reddy K. J., Bullerjahn G. S., Sherman D. M., Sherman L. A. Cloning, nucleotide sequence, and mutagenesis of a gene (irpA) involved in iron-deficient growth of the cyanobacterium Synechococcus sp. strain PCC7942. J Bacteriol. 1988 Oct;170(10):4466–4476. doi: 10.1128/jb.170.10.4466-4476.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy K. J., Haskell J. B., Sherman D. M., Sherman L. A. Unicellular, aerobic nitrogen-fixing cyanobacteria of the genus Cyanothece. J Bacteriol. 1993 Mar;175(5):1284–1292. doi: 10.1128/jb.175.5.1284-1292.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy K. J., Webb R., Sherman L. A. Bacterial RNA isolation with one hour centrifugation in a table-top ultracentrifuge. Biotechniques. 1990 Mar;8(3):250–251. [PubMed] [Google Scholar]
- Riethman H. C., Sherman L. A. Immunological Characterization of Iron-Regulated Membrane Proteins in the Cyanobacterium Anacystis nidulans R2. Plant Physiol. 1988 Oct;88(2):497–505. doi: 10.1104/pp.88.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmann G., Malkin R. Iron-sulfur centers and activities of the photosynthetic electron transport chain in iron-deficient cultures of the blue-green alga aphanocapsa. Plant Physiol. 1983 Nov;73(3):724–728. doi: 10.1104/pp.73.3.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman D. M., Sherman L. A. Effect of iron deficiency and iron restoration on ultrastructure of Anacystis nidulans. J Bacteriol. 1983 Oct;156(1):393–401. doi: 10.1128/jb.156.1.393-401.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D., Bendall D. S., Howe C. J. Occurrence of a Photosystem II polypeptide in non-photosynthetic membranes of cyanobacteria. Mol Microbiol. 1992 Jul;6(13):1821–1827. doi: 10.1111/j.1365-2958.1992.tb01354.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb R., Reddy K. J., Sherman L. A. Lambda ZAP: improved strategies for expression library construction and use. DNA. 1989 Jan-Feb;8(1):69–73. doi: 10.1089/dna.1.1989.8.69. [DOI] [PubMed] [Google Scholar]
- Webb R., Reddy K. J., Sherman L. A. Regulation and sequence of the Synechococcus sp. strain PCC 7942 groESL operon, encoding a cyanobacterial chaperonin. J Bacteriol. 1990 Sep;172(9):5079–5088. doi: 10.1128/jb.172.9.5079-5088.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]