Abstract
Elevated levels of NO produced within the central nervous system (CNS) are associated with the pathogenesis of neuroinflammatory and neurodegenerative human diseases such as multiple sclerosis, HIV dementia, brain ischemia, trauma, Parkinson's disease, and Alzheimer's disease. Resident glial cells in the CNS (astroglia and microglia) express inducible nitric oxide synthase (iNOS) and produce high levels of NO in response to a wide variety of proinflammatory and degenerative stimuli. Although pathways resulting in the expression of iNOS may vary in two different glial cells of different species, the intracellular signaling events required for the expression of iNOS in these cells are slowly becoming clear. Various signaling cascades converge to activate several transcription factors that control the transcription of iNOS in glial cells. The present review summarizes different results and discusses current understandings about signaling mechanisms for the induction of iNOS expression in activated glial cells. A complete understanding of the regulation of iNOS expression in glial cells is expected to identify novel targets for therapeutic intervention in NO-mediated neurological disorders.
Introduction
Nitric oxide (NO), a bioactive free radical, is involved in various physiological and pathological processes in many organ systems, including brain and spinal cord (17, 30). At low concentrations, NO plays a role in neurotransmission and vasodilation, while at higher concentrations, it is implicated in having a role in the pathogenesis of stroke, demyelination, and other neurodegenerative diseases (62, 98). NO is enzymatically formed from l-arginine by the enzyme nitric oxide synthase (NOS). The NOS are basically divided into two forms. The constitutive form, present in neurons (nNOS) and endothelial cells (eNOS), is a calcium-dependent enzyme (62). The inducible form (iNOS), expressed in various cell types, including astroglia and microglia of the CNS, in response to wide variety of stimuli, is, however, regulated mainly at the transcriptional level and does not require calcium for its activity (44). Astroglia and microglia in the healthy brain do not express iNOS but following ischemic, traumatic, neurotoxic, or inflammatory damage, reactive astroglia and microglia express iNOS in the mouse, rat, and human (44). NO derived from activated glial cells is assumed to contribute to oligodendrocyte degeneration in demyelinating diseases (e.g., multiple sclerosis, experimental allergic encephalopathy, and X-adrenoleukodystrophy) and neuronal death during ischemia, trauma, and neurodegenerative diseases (e.g., Alzheimer's disease, Parkinson's disease, HIV-associated dementia, Huntington's disease, and amyotrophic lateral sclerosis) (84, 94).
Therefore, characterization of intracellular pathways required to transduce the signal from the cell surface to the nucleus for the expression of iNOS in activated glial cells is an active area of investigation. Despite a large number of observations describing the expression of iNOS in glial cells, the molecular events leading to the expression of iNOS are inadequately understood. Here we circumvent observations on the regulation of iNOS in non-CNS cells and discuss recent advances about signaling mechanisms for the biosynthesis of iNOS in activated glial cells.
Inducers for Glial Expression of iNOS
As observed in other immune cells such as macrophages (87), different stimuli induce the expression of iNOS in glial cells. In the following lines, these stimuli are delineated and discussed in major functional categories to appreciate the diversity of iNOS inducers in glial cells.
Bacterial products
Although glial cells in patients with neurodegenerative and neuroinflammatory disorders probably do not come in contact with bacterial lipopolysaccharide (LPS), it is however, the first stimulus shown to induce the production of NO and expression of iNOS in microglial cells (21, 127). Subsequently, several studies have used LPS to trigger the expression of iNOS in various glial cell lines and primary astrocytes and microglia (15, 105, 109). In microglia, LPS alone is sufficient to induce iNOS. However, controversial opinions exist with regard to the role of LPS in astroglial iNOS induction. While several reports suggest that astroglial iNOS induction requires a co-stimulatory effect of IFN-γ along with LPS (15, 119, 129), another set of observations suggest that LPS alone is sufficient for iNOS expression in primary astroglial cells (45, 99, 105, 109). Interestingly, although iNOS is stimulated by LPS in rat and mouse glial cells, human glial cells do not demonstrate iNOS upregulation in response to LPS.
Glial cells respond to LPS via more than one kind of receptors. Toll-like receptor (TLR)-4, the primary receptor for bacterial endotoxin, is expressed in glial cells (18, 80). However, involvement of TLR-4 in LPS triggered iNOS upregulation is yet to be experimentally undetermined. On the other hand, the involvement of CD14, a LPS binding glycosylphosphatidylinositol-anchored receptor, is well documented in this regard (46).
In addition to LPS, bacterial DNA has been shown to stimulate iNOS expression in mouse glial cells (27, 57). TLR-9, the receptor for bacterial DNA, is expressed in glia and thus permits sensitization of these cells against the foreign DNA containing motifs of unmethylated CpG dinucleotides (27).
Viral products
Several viruses can induce iNOS in brain cells in a wide variety of species. To illustrate our point, let us consider some examples. Virulent strains of Marek's disease viruses induce iNOS in chicken brain (65). In mammals, glial iNOS is induced by several viruses, including Junin virus (in rodents) (53), Sindbis virus (in murines) (19), Theiler's murine encephalomyelitis virus (86) (in murines), and Venezuelan equine encephalitis virus (in equines) (125). Human glial iNOS is unmistakably upregulated in response to human immunodeficiency virus (HIV) intrusion in brain.
How do viruses induce iNOS in glial cells? While whole viruses are sometimes required for the effect, often, however, isolated viral products such as viral nucleic acids or proteins, are strong inducers of iNOS in glial cells. We have demonstrated that viral dsRNA induces iNOS in human astroglia via dsRNA-dependent protein kinase (PKR) pathway (4). Similarly, various proteins of viral origin also trigger iNOS expression. HIV coat protein gp41 demonstrates a steady quantitative co-relation between itself and levels of iNOS in HIV-associated dementia (HAD) (2). This coat protein alone also induces iNOS in primary cultures of mixed rat neuronal and glial cells. Furthermore, the HIV surface glycoprotein gp120 also induces iNOS in human astrocytes (140). In addition to surface proteins, viral transcription factors, like HIV-Tat, also trigger upregulation of iNOS. We have observed that overexpression of HIV-1 Tat in human astroglial cell line (U373MG) and primary astrocytes leads to significant increase in expression of iNOS (85). Similar effect of Tat has also been recorded in microglial cells (118). Moreover, engineered nonreplicating adenovirus vectors of viral origin also upregulate iNOS in rat microglia (12).
Cytokines
Cytokines are best characterized among components of innate or adaptive immune response that induce iNOS in glial cells. Many proinflammatory cytokines associated with innate immune system induce iNOS expression in both astroglia and microglia of various species. Few cytokines, such as IL-1β and IFN-γ, alone can induce iNOS in glial cells. Other cytokines (e.g., TNF-α) usually induce iNOS in conjunction with IL-1β or IFN-γ. Although IFN-γ alone is sufficient to induce iNOS in rat and mouse primary microglia (64, 138), no such effect has been reported so far in human primary microglia. In fact, we have found that different cytokines alone or in combination are also unable to induce the production of NO in human fetal microglia (unpublished observation). On the other hand, human fetal astrocytes induce iNOS upon cytokine stimulation (63). Among different cytokines tested in our laboratory and elsewhere, it has been found that only IL-1β alone or combinations involving IL-1β as a partner such as IL-1β + IFN-γ and IL-1β + TNF-α is/are capable of inducing iNOS in human primary astrocytes (58, 63).
In contrast to IL-1β or TNF-α, both of which are multimers of a single subunit, IL-12 is an interesting iNOS inducing heterodimeric cytokine. This 70 kd cytokine is composed of two subunits, p40 and p35. We have shown that IL-12 is a potent inducer of iNOS in mouse microglia (108). However, individual overexpression of p40 and p35 in BV2 microglial cells suggested that only p40 subunit, but not p35, was participating in iNOS induction (108). Consistently, we have also observed that the p40:p40 homodimer alone is capable of inducing iNOS in mouse primary microglia (108).
In addition to the above mentioned immune-response associated cytokines, other cytokines are now also known to induce iNOS. Macrophage colony stimulating factor (M-CSF), a classical hematopoietic cytokine, strongly augments iNOS expression primarily induced by amyloid-β peptide (Aβ), the etiological factor of Alzheimer's disease in BV2 microglial cells (96) and in astrocytes of hippocampal organotypic cultures (139). In addition to supplementing Aβ shock, M-CSF alone also upregulates iNOS mRNA in BV2 cells after 18 h of treatment (96).
Cell–cell contact
During diseased conditions, for example in multiple sclerosis (MS), the leaky blood–brain barrier permits infiltration of many peripheral cells into the brain. Many such cells, notably T cells, can induce iNOS expression in glial cells by establishing contact with them. For illustration of the point, let us consider the case of autoimmune T cells that lead to MS. We have previously shown that myelin basic protein (MBP)-primed T cells, which confer autoimmune reactions by recognizing self-myelin antigens, induce the expression of iNOS in mouse microglia (28). Furthermore, this study also demonstrates that iNOS induction is primarily dependent on the contact between MBP-primed T cells and microglia. Placement of MBP-primed T cells in a culture insert, where they are in close proximity but not in contact with microglia, fails to induce iNOS in microglial cells (28).
What molecular events may mediate the trigger during such contacts? A logical envisioning could involve ligand–receptor interaction of moieties exhibited on membranes of either cell type. Indeed, our experiments showed that VLA-4 integrin on the surface of neuroantigen-primed T cells and its interaction with VCAM-1 on microglial cells play an important role in contact-mediated induction of iNOS in microglia (28). However, VLA-4-VCAM-1 interaction is not the only molecular bridge representing cell–cell contact leading to iNOS expression. In the presence of IFN-γ, ligation of microglial CD40 by either cross-linking antibodies or recombinant CD40 ligand (CD154), has also led to iNOS expression in these cells (64). In light of these molecular mechanisms, we may consider cell–cell contact as a prime factor of glial expression of iNOS.
Neurodegenerative toxins
Several neurodegenerative diseases, such as AD, PD, HAD, etc, are characterized by neuronal degeneration and simultaneous activation of glial cells, leading to a local proinflammatory milieu in infarct tracts. Therefore, it is suggestive that the neurotoxic molecules may induce iNOS, an important component of glial proinflammatory response. Indeed, neurotoxins, like amyloid-β peptides (Aβ) and oxidation product of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP), induce iNOS in glial cells. During AD, toxic Aβ is formed by cleavage of amyloid precursor protein (APP) followed by aggregation of the cleaved peptides. These aggregated fibrillar Aβ peptides trigger iNOS activation in primary microglia. Primarily, fibrillar Aβ peptides activate tyrosine kinase receptor Lyn (24). In addition to Aβ, the secreted derivative of APP also triggers iNOS expression in rat primary microglia (8). Similarly, administration of MPTP in C57/BL6 mice leads to robust gliosis marked by upregulation of glial iNOS (83). A synthetic peptide consisting of amino acid residues 106–126 of the human prion protein, the endogenous misfolded villain of the Creutzfeldt–Jakob disease in humans, also induces iNOS in human microglial cells (38). Galactosylsphingosine (psychosine) accumulation, considered as the etiological cause of Krabbe disease, also upregulates iNOS expression in human astrocytes (49). In Huntington's disease (HD), iNOS level is elevated in glial cells in region of the degenerating infarct (22). Similarly, during amyotrophic lateral sclerosis (ALS), strong iNOS immunoreactivity is recorded in activated astrocytes (7). However, in both these cases, the exact inducer of glial iNOS is not yet experimentally verified.
Neurodegenerative insults
In addition to the above mentioned neurodegenerative diseases, glial iNOS is expressed profusely during several other neurodegenerative insults. Ischemic shock induces iNOS in mice microglia (67, 114). Chronic and acute spinal cord injury also leads to iNOS expression by local glial cells (11). One of the major inducers of iNOS during these insults is oxidative stress generated free radicals (detailed subsequently). For example, expression of glial iNOS due to administration of quinolinic acid is appreciably reversed by co-administration of pyruvate, a glycolysis end product with antioxidant activity (123).
In the following lines, elaboration on the mechanism of iNOS regulation by these inducers is attempted.
Regulation of Glial iNOS Expression
We do not have ample evidence to consider regulation of iNOS in glial cells (astroglia and microglia) in a different light than what is used to define the same in other cell types. However, a couple of studies indicate that the regulation of iNOS in astroglia is a distinct affair from the regulation of this gene in other immune cells in periphery such as macrophages (105, 109). Moreover, we cannot reach a conclusion as microglia, the other glial cells, are not considered in these studies. Therefore, we will restrict ourselves from mentioning glia-specific regulatory events for the expression of iNOS and discuss mechanisms that are known to control the expression of iNOS in glial cells. As expected, glial iNOS can be regulated at the transcriptional, posttranscriptional, translational, and posttranslational level.
Transcriptional regulation
As observed in case of other inducible genes, iNOS is also regulated mainly at the transcriptional level. However, transcriptional regulation of iNOS is very complex. Several intracellular signaling cascades have been found to control the expression of iNOS in glial cells at the transcriptional level. In the following lines, we first describe the iNOS promoter and subsequently elaborate on various transcription factors and signaling pathways involved in its induction in glial cells. A general overview of the same is presented in Table 1.
Table 1. Major Signlaing Pathways Triggered by Various INOS Inducers in Glial Cells.
| Inducer Class | Inducer | Major pathway triggered |
|---|---|---|
| Viral and bacterial products | LPS | CD14 → ERK1/2 and p38 → NF-κB → iNOS |
| Bacterial DNA | TLR-9 → MyD88 → p38 → iNOS | |
| viral dsRNA | Receptor (?) → PKR → NF-κB → iNOS | |
| Viral Tat | Receptor (?) → ERK2 → C/EBP → iNOS | |
| Cytokines | IL-1β | IL-1βR → p38 → NF-κB → iNOS |
| IFNγ | IFNγR → JAK → STAT → iNOS | |
| IL-12 (p40)2 | Receptor (?) → NF-κB → iNOS | |
| Cell–cell contact | Engagement of surface molecules | VLA4-VCAM-1 → C/EBP → iNOS |
| CD40-CD40L → NF-κB → iNOS | ||
| Neurotoxins | Fibrillar Amyloid-β | Lyn (?) → NF-κB (?) → iNOS |
| Secreted APP | Receptor (?) → JNK/ p38 → iNOS | |
| 106–126 aa of hPrion | Receptor (?) → p38 → NF-κB → (TNFα↑) → iNOS |
Promoter of iNOS
Cloning of iNOS promoter from different species has paved the way for investigating molecular mechanisms required for transcriptional regulation of the iNOS gene. Although in the mouse iNOS gene, about 1.7 kb promoter exhibits full inducibility to a mixture of LPS and IFN-γ (147), a 3.2 kb rat iNOS promoter was required for full inducibility (149). On the other hand, functional promoter elements that regulate cytokine-inducible human iNOS expression are located upstream of 3.8 kb (31). According to Taylor et al. (136), a 7.2 kb promoter segment of the human iNOS gene confers partial inducibility in response to cytokines. A promoter segment of 8.3 kb or 16 kb is required for higher level of activation in non-CNS cells (78, 135) suggesting that the regulation of human iNOS promoter is very complex. For example, promoters of several inflammatory response genes, including cell adhesion molecules ELAM-1, VCAM-1, and ICAM-1 and the cytokines IL-1, IL-6, and IL-8 contain only one or two proximally-located functional NF-κB binding sites. The human iNOS gene instead houses eight NF-κB binding sites that are located more than 5.2 kb upstream of the TATA box (136) and many of them are located within a segment of ∼800 bp. Although functional relevance of the presence of multiple functional NF-κB binding sites is poorly understood, it is believed that the number of NF-κB binding sites in human iNOS promoter may influence the intensity of the response to NF-κB depending on the concentration of this transcription factor in a particular cell type. As for example, low concentrations of NF-κB would be expected to evoke only a minimal response; however, with increasing levels of NF-κB translocating into the nucleus, more NF-κB sites would become occupied and a greater response elicited. To further complicate the situation, it has been predicted that the 5′-flanking region of the human iNOS gene is more than 30 kb and may contain other positive regulatory elements (136).
Although most of the studies related to manipulation of iNOS promoter have been performed in non-CNS cells, a few studies have also registered the activation of iNOS promoter in mouse and rat glial cells (13, 106, 117, 143). In recent past, our lab has mainly concentrated on the activation of human iNOS promoter in human U373MG astroglial cells and primary astrocytes. We have observed that a number of stimuli (e.g., IL-1β, the combination of IL-1β and IFN-γ, double-stranded RNA, and HIV-1 Tat) are capable of activating a 7.2 kb human iNOS promoter by three- to fourfold in human primary astroglia and U373MG astroglial cells (4, 85, 102). Our recent studies have shown that IL-1β or cytokine combinations involving IL-1β as a partner induce the activation of an 8.8 kb human iNOS promoter by 5.5- to 8.2-fold in human primary astroglia (63). This increase in iNOS promoter activity could be due to the presence of extra positive regulatory elements within 7.2–8.3 kb human iNOS promoter, which is responsible for higher promoter activity in response to cytokines (63).
Involvement of different transcription factors
Among all the transcription factors working in concert to transactivate the iNOS promoter, nuclear factor kappaB (NF-κB) p50:p65 is literally the prima donna. The presence of multiple consensus sequences (kappaB elements) in the promoter region of human, rat, and mouse iNOS for the binding of NF-κB and the inhibition of iNOS expression in human, rat, and mouse glial cells with the inhibition of NF-κB activation by PDTC (111) or NBD peptide (29) establishes an essential role of NF-κB activation in the induction of iNOS. All different inducers of iNOS have been shown to recruit NF-κB in a wide variety of cell types via one or more kinase pathways (see next section). Activated kinase pathway(s) phosphorylate the NF-κB p50:p65 arresting protein, inhibitory kappaB (IκB) in the cytosol, thereby subjecting it for ubiquitination and subsequent proteosomal degradation. This liberates the p50:p65 heterodimer to enter the nucleus and bind kappaB elements in the iNOS promoter. Interestingly, rat and mouse iNOS promoter harbor two distinct regions of kappaB elements. One of these is proximal, within ∼100 nucleotides of the initiation site and the other is about −1,000 bp distal from it on 5′-flanking region (Table 2). A similar scenario is also present in human iNOS promoter with a comparable proximal kappaB element. However, the distal region spans between −5.8 and −7.2 kb and is composed of cytokine sensitive multiple NF-κB binding elements (136).
Table 2. Involvement of Different Transcription Factors in the Activation of iNOS Promoter.
| Transcription factor | Recruiting trigger | Species specificity | Important Promoter Binding region | Remarks |
|---|---|---|---|---|
| NK-κB p50:p65 | LPS, viral products, cytokines, oxidative stress, cell–cell contact, neurotoxins, etc. | R, M, H | MP: (i) −76 to −85(146) | Recruitment to promoter invariably leads to promoter transactivation |
| (ii) −974 to −960(132) | ||||
| RP: (i) −107 to −98 | ||||
| (ii) −965 to −956(37) | ||||
| HP: (i) −115 to −106(101) | ||||
| (ii) many others(136) | ||||
| C/EBP | LPS, viral products, CD40 ligation, cAMP, hypoxia IL-1β, dsRNA, glucose metabolites. | R, M, H | MP: (i) −153/−142(36) | Co-activator modulation? |
| RP: (i) −155 to −163(37) | ||||
| HP: (i) −205 to +88 (several)(75) | ||||
| STAT-1 | IFN-γ | R, M, H | MP: (i) −934 to −942(48) | Depending on stimuli, GAS elements may impart opposite effect on promoter activity. |
| RP: (i) −936 to −928(37) | ||||
| HP: (i) around −5.2 kb (STAT specific) | ||||
| (ii) around −5.8 kb (STAT/NF-κB)(47) | ||||
| IRF-1 | IFN-γ | R, M | MP: (i) −901 to −913(51) | Species specific? |
| (ii) −913 to −923(89) | ||||
| RP: (ii) −929 to −881(131) | ||||
| HP: ? | ||||
| AP-1 | Galactosylsphingosine, cytokines | R (?), M, H | MP: (i) −1125*(71) | Controversial role. May induce or suppress iNOS promoter. |
| HP: (i) −5115 | ||||
| (ii) −5301(78) |
Along with NF-κB, CCAAT/enhancer-binding protein (C/EBP) is another transcription factor of prime importance in induction of glial iNOS and is induced in these cells by a wide array of stimuli (Table 2). The C/EBPs are a family of six basic leucine zipper transcription factors (81). Among them, C/EBPβ (4, 13, 63, 85) and C/EBPδ (143) have been reported to regulate in glial iNOS induction. C/EBPβ and C/EBPδ may form homo- or heterodimer between themselves. In our studies, we have found C/EBPβ to be an obligatory factor in regulation of iNOS induction in response to IL-1β + IFN-γ (63), dsRNA (4), HIV-tat (85), and contact with neuroantigen primed T-cell (28). Similar conclusions have also been reached by other groups in studies with glucose dependent induction of iNOS (143). What renders C/EBP such an unavoidable critical role in iNOS regulation? The answer probably lies in their capacity to recruit and phosphorylate histone acetyl-transferase (HAT) co-activators, like p300 and CREB binding protein (CBP). Signal-induced nuclear translocated C/EBPβ has been shown to recruit and phosphorylate p300-HAT (126), while C/EBPδ performs similar modifications on CBP-HAT and recruits it to the active transcriptional complex (77). Abolishing this C/EBP dependent phosphorylation of co-activators by introducing specific mutations attenuates the transactivation of C/EBP contingent genes, probably by interfering with formation of active transcriptosome. Interestingly, critical binding sites for C/EBP are located very near to transcription initiation site in all three mammalian iNOS promoter (Table 2).
Besides kappaB and C/EBP binding elements, all mammalian iNOS promoters house the STAT-1α (signal transducers and activators of transcription-1α) binding sequences, called GAS element (Table 2). Although STAT-1α has been found to inhibit NF-κB activity with respect to iNOS induction in some cases (47), in glial cells Jak–STAT and NF-κB pathways synchronize at least in response to IL-1β + IFN-γ treatment (63). In addition to STAT-1α, IFN-γ also activates interferon regulatory factor-1 (IRF-1) as a secondary response. So far, interferon-stimulated responsive element (ISRE), the DNA binding sequence for IRF-1, has been identified in rat and mouse iNOS promoter where two ISRE were found to be crucial (89) (Table 2). IRF-1 has been shown to physically bend iNOS promoter region, thereby facilitating its activation (124). This is a unique property and has not been reported for other transcription factors. Although consensus ISRE is not yet reported in human iNOS promoter, however, several related elements have been detected in it (23). Is IRF-1 involved in glial expression of iNOS? In response to LPS and IFN-γ treatment, mixed glial cells from IRF-1 (–/–) mice express reduced iNOS mRNA in comparison to their wild-type litter mates (43). It is important to note here that ablation of IRF-1 did not abolish iNOS expression completely, suggesting a supplemental role for IRF-1 in iNOS expression. Strangely, the involvement of IRF-1 is not yet reported in human glial cells. We have observed that IL-1β + IFN-γ, the combination that efficiently induces iNOS in human astrocytes, is unable to induce ISRE-dependent reporter activity in the same cells (102). This suggests a probable species specific function of this transcription factor.
One more transcription factor that is widely studied in this regard is activator protein-1 (AP-1). The 8.3 kb human iNOS promoter has a couple of consensus AP-1 binding elements (Table 2). While the above-mentioned transcription factors are now widely accepted to impart positive influence to iNOS transactivation, AP-1 harbors controversy regarding its exact role in this regard in glial and nonglial cells. In a study with galactosylsphingosine in rat astroglial cells, Giri et al. utilized AP-1 binding element containing decoy DNA and showed that AP-1 plays a positive role in maintaining high level of iNOS mRNA after galactosylsphingosine treatment (49). This group has also suggested a positive role for AP-1 in Aβ-treated glial cells (5). Observations in our laboratory offer a slightly variant version. In a study with human primary astrocytes and different cytokines, we have found that AP-1 is involved in IL-1β, but not (IL-1β + IFN-γ)-induced expression of iNOS (63). IL-1β or IFN-γ alone significantly induces the transcriptional activity of AP-1. However, a combination of these two cytokines, that stimulates iNOS promoter maximally, completely reverses the transcriptional activity of AP-1 (63). This combined treatment, however, does not block the DNA binding activity of AP-1. We therefore believe that, although activation of AP-1 may represent an essential step in iNOS regulation in glial cells under certain conditions, it is not absolutely required for glial expression of iNOS by other stimuli.
Transcriptional regulation by tyrosine kinases
Tyrosine kinase super family is comprised of Janus kinase (JAK) family, src family, mitogen-activated protein kinase kinase (MAPKK) family, and receptor-linked tyrosine kinase family. In general, broad-spectrum tyrosine kinase inhibitors (e.g., genistein and herbimycin A) are capable of inhibiting almost every family member. Several evidences indicate that these broad-spectrum tyrosine kinase inhibitors inhibit LPS-and cytokine-mediated expression of iNOS in glial cells (39, 76) suggesting the possible involvement of tyrosine kinase(s) in glial expression of iNOS. Among all the tyrosine kinases, the role of JAK in the expression of iNOS in glial cells has been extensively studied. JAK–STAT signaling, originally identified as the signaling pathway for IFNs, mediates the immune responses of various cytokines as well as the actions of many growth factors and hormones. Usually the binding of a ligand to its receptor induces the assembly of an active receptor complex and subsequent phosphorylation of the receptor-associated JAKs (JAK1, JAK2, and JAK3) and tyrosine kinase 2 (Tyk2). Phosphorylated JAKs lead to the phosphorylation of STATs, which in turn are released from the receptor complex and form homo- or heterodimers (Fig. 1). These dimers translocate to the nucleus where they directly bind to the promoter region of several inflammation-associated genes including iNOS. Consistently, Xie et al. (147) has provided evidence that the promoter region of mouse iNOS gene contains the IFN-γ activation site (GAS) and that GAS is activated by tyrosine phosphorylated STAT1α. According to Kitamura et al. (70), the activation of JAK2 is mainly involved in IFN-γ-induced expression of iNOS in glial cells. In fact, IFN-γ induces tyrosine phosphorylation of STAT1α but not STAT1β via JAK2 and that tyrosine phosphorylation of STAT1α seems to be essential for IFN-γ-induced expression of iNOS in glial cells (33). These observations suggest that IFN-γ-induced activation of JAK2 phosphorylates STAT1α that in turn binds to GAS elements in the promoter of iNOS. Although inhibitors of tyrosine kinase inhibit LPS-induced expression of iNOS in glial cells (76, 128), LPS-induced expression of iNOS does not involve the activation of JAK–STAT signaling pathway (100).
FIG. 1. Regulation of iNOS by JAK–STAT pathway.
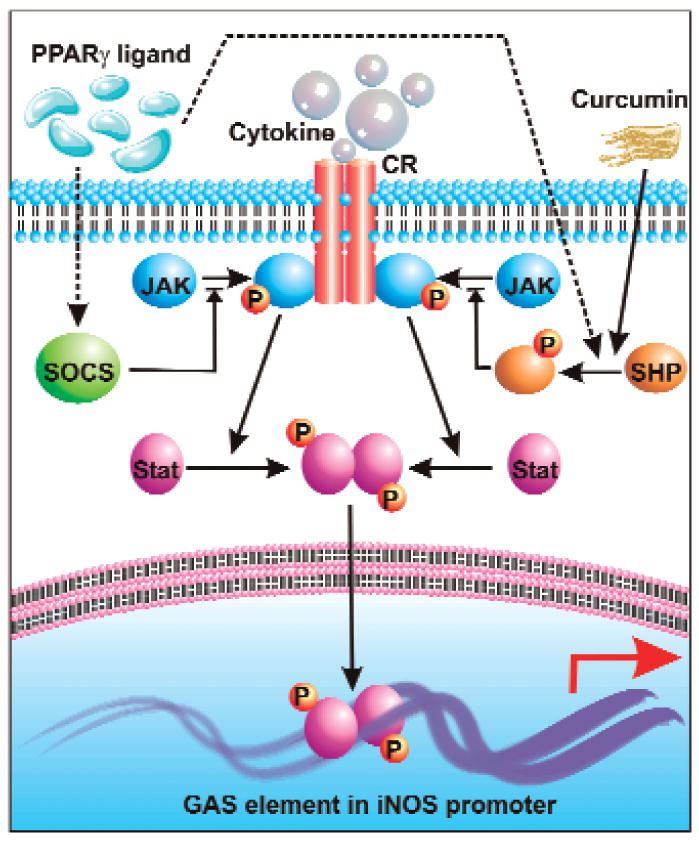
Ligand-mediated engagement of receptor tyrosine kinases leads to their auto-activation and permits recruitment and phosphorylation of JAK. Thereafter, receptor bound JAK mediates phosphorylation, dimerization, and activation of STAT. These active dimers translocate to the nucleus and bind to GAS elements in iNOS promoter. SHP-1 and SHP-2 can block recruitment of JAK to the RTK and thus prevent activation of the JAK–STAT pathway. This scheme is utilized by curcumin {diferuloylmethane, 1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione} and PPARγ ligands, as they can mediate activation of SHP. PPARγ ligands can also upregulate SOCS expression, which subsequently blocks recruitment of JAK to the RTK.
In addition to coupling the JAK–STAT1α pathway for the regulation of iNOS, tyrosine kinases may also regulate iNOS expression via activation of NF-κB (100). Although tyrosine kinase inhibitors have been shown to inhibit the activation of NF-κB in glial cells, the mechanism by which tyrosine kinase(s) control the activation of NF-κB is poorly understood.
Transcriptional regulation by SOCS
Recent studies show that cells can employ a family of proteins called suppressors of cytokine signaling (SOCS) to challenge proinflammatory signaling pathways of various cytokines. This family of proteins is now considered as important regulators of normal immune physiology and immune disease (82). In general, SOCS are present in cells at very low levels. However, SOCS are rapidly transcribed upon exposure of cells to certain stimuli. Subsequently several studies indicate that SOCS can negatively regulate the response of immune cells either by inhibiting the activity of JAK or by competing with signaling molecules for binding to the phosphorylated receptor (3). For example, both SOCS1 and SOCS3 are capable of binding JAKs to suppress their tyrosine kinase activity (113). According to Park et al. (113), 15d-PGJ2 and rosiglitazone, activators of PPAR-γ, induce the transcription of SOCS1 and SOCS3 to inhibit the activity of JAK1 and JAK2 in rat primary astrocytes and microglia (Fig. 1). Therefore, 15d-PGJ2 and rosiglitazone reduce the phosphorylation of STAT1 and STAT3 and block the expression of iNOS in activated glial cells. These results suggest that upregulation of SOCS may represent a critical step for suppressing expression of iNOS in glial cells via negative regulation of the JAK–STAT pathway.
Transcriptional regulation by mevalonate metabolites
The mevalonate pathway is involved in the biosynthesis of cholesterol in cytoplasm from acetyl-CoA (Fig. 2). However, the idea of investigating the role of the mevalonate pathway in the regulation of iNOS came from the fact that intermediates of this biochemical pathway are isoprenoids, which are known to play an important role in isoprenylating small G proteins like Ras and Rac (52). Upon isoprenylation, these G proteins go to the membrane and transduce several intracellular signaling pathways. Therefore, drugs capable of reducing cholesterol biosynthesis also inhibit isoprenylation and activation of small G proteins (68). Interestingly, Pahan et al. (110) have shown that lovastatin, an FDA-approved drug for hypercholesterolemia, inhibits the activation of NF-κB and the expression of iNOS in LPS-stimulated rat primary astrocytes. In fact, this landmark finding has added a new dimension to statin research. Nowadays, statin drugs are being widely considered as potential therapeutic agents against various neuroinflammatory and neurodegenerative disorders. The same study also shows the antiinflammatory property of another mevalonate pathway inhibitor, sodium phenylacetate (NaPA), an FDA-approved drug for hyperammonemia in children. Similar to lovastatin, NaPA also inhibits the activation of NF-κB and the expression of iNOS in glial cells (110). As lovastatin inhibits HMG-CoA reductase, both mevalonate and farnesyl pyrophosphate (FPP) are capable of reversing the inhibitory effect of lovastatin on iNOS expression and NF-κB activation. On the other hand, NaPA inhibits mevalonate pyrophosphate decarboxylase downstream of mevalonate synthesis, therefore, FPP but not mevalonate, blocks the inhibitory effect of NaPA (Fig. 2). However, addition of ubiquinone and cholesterol to astrocytes does not prevent the inhibitory effect of lovastatin and NaPA. These results suggest that depletion of farnesyl pyrophosphate, rather than end products of mevalonate pathway, is responsible for the observed inhibitory effect of lovastatin and NaPA on the expression of iNOS.
FIG. 2. Crosstalk between cholesterol synthesis and iNOS gene expression.
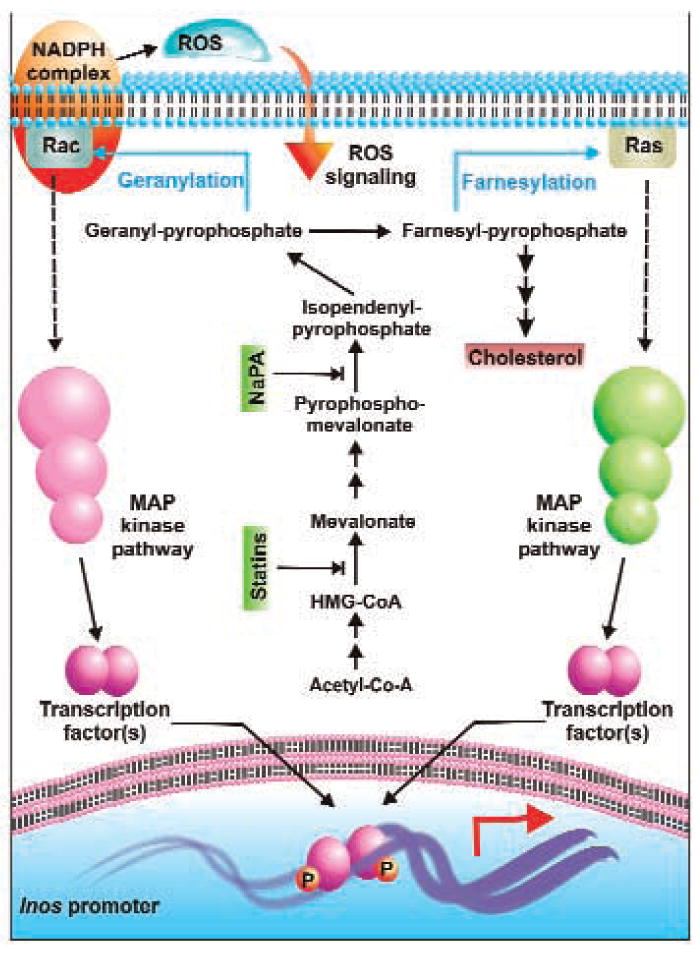
Geranyl-pyrophosphate and farnesyl-pyrophosphate, generated by condensation of the mother carbon skeleton of isopendenyl pyrophosphate, modify membrane-coupled Rac and Ras, respectively, leading to their activation. Subsequently, MAP kinase pathways are employed to activate specific transcription factors, which then bind to their elements in the iNOS promoter and transactivate them. iNOS expression blockers may impede this pathway by interfering at specific points in the cholesterol synthesis pathway. Furthermore, activation of Rac also leads to activation of the NADPH complex. Thus, ROS is generated, which diffuses back into the cell to mediate its own signal is augmenting iNOS expression.
Suppression of LPS-induced activation of NF-κB and expression of iNOS in glial cells by farnesyltransferase inhibitors (97, 103) suggests an important role of farnesylation reaction in the regulation of iNOS gene. Consistent to a role of farnesylation in the activation of p21Ras, a dominant–negative mutant of p21Ras (S17N) also attenuated activation of NF-κB and expression of iNOS in rat and human primary astrocytes (103). Recently, another well-executed study by Cordle and Landreth (25) also indicates that statins inhibit fibrillar Aβ-induced expression of iNOS in mouse BV-2 microglial cells by inhibiting isoprenylation of Rac. Taken together, these studies suggest that mevalonate metabolites regulate the expression of iNOS in glial cells via modulating isoprenylation of Ras and Rac.
Transcriptional regulation by MAP kinases
Several studies have now identified involvement of all three MAP kinase (ERK1/2, p38, and JNK) pathways in iNOS regulation. In the following lines, we will restrict our discussion to findings relevant to glial iNOS regulation only.
Regulation by MAP kinases in astroglia
A number of studies with inhibitors of MAP kinases or with their dominant negative forms have revealed distinct as well as overlapping roles of all three MAP kinases in glial iNOS machinery in response to a wide array of stimuli. In rat astrocytes, combined treatment of LPS + IFN-γ induces p38 and ERK1/2 pathways (15). Similarly, treatment with a combined cytokine regimen of TNF-α + IL-1β utilizes the MEK–ERK pathway (88). Uniquely, the same study also reported that blocking p38 MAPK with its inhibitor amplified nitrite accumulation, suggesting a gain of function for iNOS in the absence of potent p38. This observation stands in some degree of isolation and may represent an inducer-specific effect as rat astrocytes require p38 activity for iNOS expression in response to other stimuli like Taxol (26) and activation of upstream kinase TGF-β-activated-kinase (TAK) (14). Along the same lines, p38 MAP kinase pathway is also a major mediator of human astroglial iNOS expression in response to IL-1β + IFN-γ (our unpublished observation), IL-1β (58), and dsRNA (4).
More often than not, p38 MAP-kinase pathway functions in parallel with JNK pathway. In rat astrocytes, dual activity of JNK and p38 has been observed in several cases (26, 14). In human astrocytes, similar trend is also reported for iNOS induction by IL-1β (58). However, when co-administered with IFN-γ, the combination (IL-1β + IFN-γ), triggers iNOS in a p38-sensitive (our unpublished observation) but JNK-insensitive way (63). Similarly, we have observed that HIV-1 Tat induces iNOS in human astroglial cells in a (MEK–ERK)-responsive but p38-nonresponsive way (85). Taken together, this clutter of diverse observations suggests that signal transduction for astroglial expression of iNOS is conveyed by all three MAP kinase pathways. However, they are employed in various combinations depending mainly upon the inducing stimuli.
Regulation by MAP kinases in microglia
Literature in this area is restricted to work done in rat primary microglia or in BV2 mouse secondary microglial cell lines. Like astrocytes, different stimuli utilize one or more of the three MAP kinase pathways in microglia. For example, p38 and JNK pathways are utilized simultaneously during iNOS induction by hyaluronan (142), soluble APP (16), adenoviral vectors (12), and TAK1 (14) in microglial cell lines. However, iNOS expression due to hypoxic shock in BV2 cells is sensitive only to p38 inhibitor and not to inhibitors of JNK (114). LPS, on the contrary, utilizes a different combination of MEK–ERK and p38 pathways to upregulate iNOS (15). Involvement of ERK1/2 pathway in LPS trigger is further confirmed by a study with parthenolide, a sesquiterpene lactone, where LPS-induced microglial iNOS upregulation is attenuated by parthenolide by blocking ERK1/2 activity (42).
Regulation of transcription factors by MAP kinases
MAP kinases convey iNOS upregulating signal by activating different transcription factors, which then translocate to the nucleus and directs transcription initiation at iNOS promoter. In light of differential species or cell type-specific regulation of iNOS promoter, several permutations and combinations are viable in producing the desired effect. However, to represent the situation in glial cells, we have attempted to knit a flow-diagram representing transcription factor specificity for several MAP kinase pathways (Fig. 3). A quick glance at this figure reveals the central role of p38 MAPK in this regard. In rat astroglia, p38 has been shown to regulate NF-κB, C/EBP, and ATF-2 (13). In human primary astrocytes, we have seen a significant loss of transcriptional activity for NF-κB, C/EBP, STAT-1, and AP-1 in the exclusive presence of a p38 MAPK inhibitor (63, unpublished data). However, p38 may not be involved in response to several stimuli. For example, HIV-1 Tat-induced iNOS expression in human astrocytes is dependent on the ERK pathway (85). In this case, inhibitor for the kinase (PD98059) and dominant–negative mutant of ERK-2 blocked iNOS expression by interfering with C/EBPβ, but not NF-κB, activity. In contrast to these two MAPK pathways, the JNK pathway triggers the activation of AP-1 primarily in response to cytokines. Blocking this pathway attenuates IL-1β-, but not (IL-1β + IFN-γ)- induced iNOS expression in human primary astrocytes (63).
FIG. 3. General overview of MAP-kinase pathways (MKP), transcription factors, and iNOS transactivation.
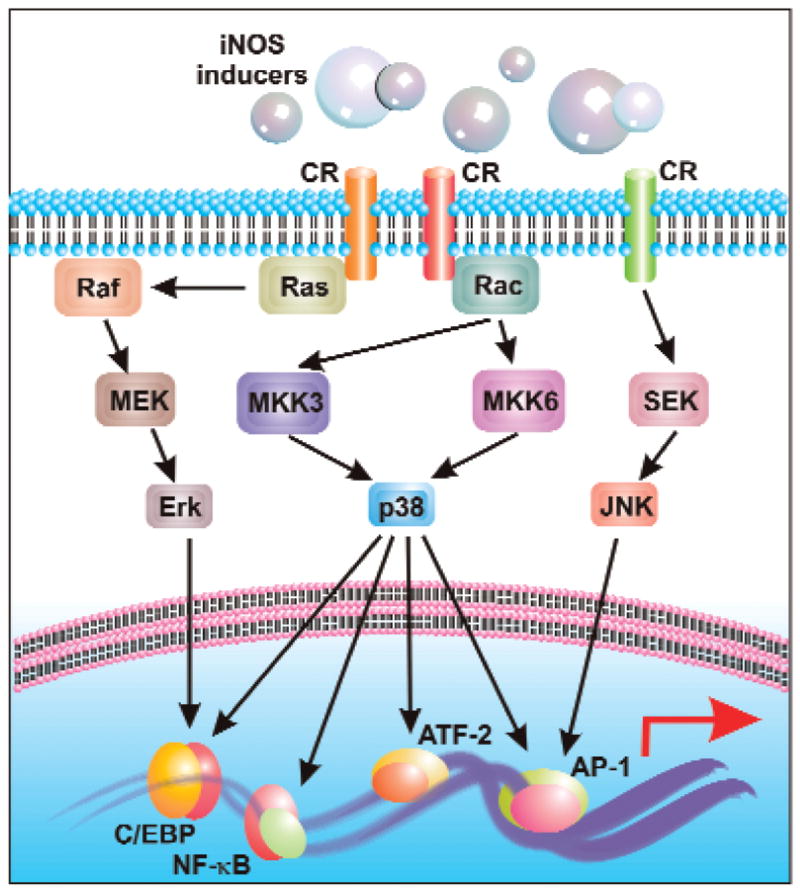
Activation of cytokine receptors induces a downstream signal, which is mediated by one or more of the three MKPs. Several transcription factors act downstream of specific MKP, which then translocate to the nucleus to transactivate iNOS. This figure indicates the central role of p38 MKP in iNOS induction.
Transcriptional regulation by cyclic AMP
cAMP, one of the most important cellular second messengers, functions through protein kinase A (PKA), which is an integral constituent of the protein kinase cascade that couples a number of extracellular signals to variety of cellular functions. Subsequently, it has been shown to regulate the induction of iNOS in various cell types. For example, cAMP induces the expression of iNOS in LPS- and cytokine-stimulated glomerular mesangial cells (95), vascular smooth muscle cells (73), cardiac myocytes (60), murine 3T3 fibroblasts (72), and rat peritoneal macrophages (130). In these studies, the increase in cAMP level resulting due to inhibition of phosphodiesterase (an enzyme that degrades cAMP), or due to an exogenous supply of cAMP derivatives or compounds that enhance intracellular levels of cAMP induce iNOS. In contrast to these results, cAMP inhibits the expression of iNOS and the production of NO in LPS- or cytokine-stimulated rat primary astrocytes or C6 glial cells (105). The reciprocal relationship between the activation of PKA and the expression of iNOS by forskolin, 8-Br cAMP, and Sp-cAMP supports the conclusion that cAMP negatively regulates the expression of iNOS in astrocytes (105). One potential intracellular target of LPS signaling in cells is the activation of the MAP kinase pathway. It has been shown by several groups that augmentation of intracellular cAMP blocks the signaling pathway from Ras to MAP kinase in cells such as fibroblasts and fat cells by phosphorylation of Raf (an upstream member of MAP kinase pathway) (54, 145). However, one recent study (144) with rat primary astrocytes and C6 glial cells shows that cAMP in fact inhibits the activation of p38 MAP kinase, the kinase that is involved in the activation of NF-κB in most cases. Different neurotransmitters are known to increase the level of cAMP in the CNS. Consistently, norepinephrine has been reported to inhibit the expression of iNOS in glial cells via the cAMP–PKA pathway. Feinstein and co-workers have observed that NE actually increases the expression of IκBα, the inhibitory subunit of NF-κB, to arrest the active NF-κB heterodimer in the cytosol. Because in all these studies, the final outcome is inhibition of NF-κB activation by cAMP, it is possible that increasing cAMP may inhibit astroglial activation of NF-κB at both the steps. Apart from NF-κB, p38 is also involved in the activation of AP-1, C/EBPβ, and ATF-2, the transcription factors that positively regulate the transcription of iNOS gene in astrocytes. Therefore, cAMP is expected to inhibit astroglial expression of iNOS via downregulation of p38-coupled proinflammatory transcription factors. In addition, the role of cAMP-response element binding protein (CREB) in cAMP-mediated downregulation of astroglial iNOS cannot be over-ruled. Because cAMP is capable of activating CREB via PKA-mediated phosphorylation and the promoter of iNOS contains CRE, it is possible that cAMP–PKA may downregulate the expression of iNOS via CREB. Therefore, it will be interesting to explore the role of CREB, if any, in expression of astroglial iNOS.
On the other hand, studies regarding the role of cAMP–PKA in the induction of iNOS in microglia indicate differential regulation based on stimuli. For example, according to Fiebich et al. (41), elevation of cAMP inhibits LPS-induced microglial expression of iNOS. Consistently, another study by Minghetti et al. (93) also shows downregulation of LPS-induced microglial expression of iNOS by PGE2, forskolin, and dibutyryl cAMP. On the other hand, a different set of inducers such as, amyloid-β peptides, plasminogen, and gangliosides induce microglial expression of iNOS via cAMP–PKA (92, 120). Therefore, a detailed analysis of regulatory mechanisms by which the cAMP–PKA pathway crosstalks with the transcriptional machinery for iNOS in two different glial cells is required to assess activators of cAMP–PKA pathway as possible therapeutics.
Transcriptional regulation by reactive oxygen species (ROS)
Once ROS are produced, these multipotent diffusible molecules are capable of carrying out many signal transduction processes of various extracellular stimuli. Consistent with their versatile cellular functions, ROS also regulate the expression of iNOS in different cells including glial cells. N-acetyl cysteine (NAC) is a cell-permeable analog of cysteine, the precursor of glutathione, the molecule that plays an important role in maintaining cellular redox homeostasis. Studies have revealed that NAC and other antioxidants like pyrrolidine dithiocarbamate (PDTC) and lycopene are potent inhibitors of production of NO and the expression of iNOS in rat primary astrocytes, microglia, and C6 glial cells (111, 112). Recent studies have identified the ROS-producing molecule in activated glial cells. For example, Pawate et al. (116) have defined a critical role of NADPH oxidase in (LPS + IFN-γ)-induced expression of iNOS in glial cells. They have shown that stimulation of rat primary astrocytes and microglia leads to rapid activation of NADPH oxidase and release of ROS followed by the expression of iNOS. Consistently, attenuated induction of iNOS is observed in a microglial cell line stably transfected with a mutant form of Phox subunit (i.e., p47Phox W193R) and in primary astrocytes derived from gp91Phox-deficient mice (116). Furthermore, inhibition of (LPS + IFN-γ)-induced expression of iNOS and production of NO by exogenous addition of catalase but not superoxide dismutase suggests that H2O2 is the ROS coupled to the induction of iNOS. Recently, Cordle and Landreth have also shown that Rac1-dependent activation of NADPH oxidase and production of superoxide play an essential role in fibrillar Aβ-induced expression of iNOS in microglial cells (25). Because activation of NF-κB is involved in the expression of iNOS and antioxidants and inhibitors of NADPH oxidase suppress the activation of NF-κB in glial cells (91), ROS are believed to regulate iNOS expression via NF-κB. However, the involvement of other transcription factors in ROS-mediated induction of iNOS in glial cells cannot be ruled out as many such factors are redox-sensitive in other cell types.
Transcriptional regulation by ceramide
Several extracellular stimuli have been shown to couple the sphingomyelin–ceramide pathway that eventually leads to important biochemical and cellular responses, including apoptosis (55, 74). This pathway is initiated by the activation of neutral sphingomyelinase (NSMase) that hydrolyzes membrane sphingomyelin to ceramide and phosphocholine. Eventually, ceramide has emerged as a second messenger molecule that mimics some of the cellular effects of different cytokines, neurotoxins, apoptotic inducers, and bacterial and viral products in terminal differentiation, apoptosis, and cell cycle arrest (151). Although sphingomyelinase (SMase) increased the cellular levels of ceramide, either SMase or cell-permeable ceramide analog alone was unable to induce iNOS in rat primary astrocytes (107). However, incubation of LPS or cytokine-stimulated astrocytes with SMase, cell-permeable ceramide analogs (C2- or C6-ceramide) or inhibitor of ceramidase (N-oleoyl ethanolamine) led to a time- and dose-dependent increase in the expression of iNOS in rat astrocytes and C6 glial cells (107). The ability of ceramide to potentiate the LPS- or cytokine-mediated activation of NF-κB activation indicates that sphingomyelin–ceramide signaling events augment astroglial expression of iNOS via increasing the activation of NF-κB (107). Inhibition of iNOS expression in (LPS + ceramide)-activated astrocytes by antioxidants and inhibitors of farnesyl-transferase and MEK suggested the possible involvement of redox-sensitive Ras-ERK pathway in the activation of NF-κB and the expression of iNOS (107). Consistently, a recent well-executed study by Singh and colleagues (112) has identified lactosylceramide as an important mediator of expression of iNOS in (LPS + IFN-γ)-activated primary rat astrocytes. They have delineated that LPS/IFN-γ induces the production of Lac-Cer that in turn activates Ras–ERK pathways via redox-sensitive pathway. This Ras–ERK pathway couples to the activation of NF-κB and hence the induction of iNOS in astrocytes.
Transcriptional regulation by phosphatidyli-NOSitol-3 kinase (PI-3K)
PI-3K is a lipid kinase that phosphorylates 3′-OH position of the inositol ring of inositol phospholipids, producing PtdIns(3)P, PtdIns(3,4)P2, PtdIns(3,4,5)P3. The prototypical class-I PI-3K comprises a p110 catalytic subunit and a p85 regulatory subunit (three mammalian subtypes: α, β, and γ). As per a well-accepted model (20), the p110 subunit is recruited to the Src homology 2 (SH2) domains in the cytoplasmic fraction of an engaged tyrosine kinase receptor by a similar domain in the adapter p85 subunit. Thus, transported to a membrane proximal position, p110 performs its catalytic activity on lipid residues of the plasma membrane. Although this kinase activity is known to initiate several positive cellular responses, inhibition of the PI-3K pathway induces/stimulates LPS or cytokine induced iNOS expression in glial cells. Treatment of C6 glial cells and rat primary astrocytes with specific inhibitors of PI-3K (wortmannin and LY294002) induces the expression of iNOS in LPS- or cytokine-stimulated C6 glial cells or stimulates the expression of iNOS in rat primary astrocytes. Similar effects are seen by overexpression of dominant–negative mutant of adapter p85α in these cells (106). Along the same lines, expression of a catalytically active p110 subunit of PI 3-kinase but not that of a kinase-deficient mutant of p110 induced an increase in PI 3-kinase activity and inhibited cytokine-induced production of NO and expression of iNOS (104). Although the exact mechanism of PI-3K induced block is not understood at this point of time, it is known that these inhibitory effects are not contingent on MAP kinase pathways or activation of NF-κB (104,106).
Transcriptional regulation by protein phosphatases
Transient modulation of protein phosphorylation and dephosphorylation is a major mechanism of intracellular signal transduction pathways triggered by different extracellular stimuli. Because proteins are phosphorylated on serine, threonine, and tyrosine residues, cells are naturally armed with two different groups of enzymes that dephosphorylate these residues. Phosphoserine threonine protein phosphatases (PP1, PP2A, PP2B, PP2C) are entitled to dephosphorylate phosphoserine and phosphothreonine residues. On the other hand, phosphotyrosine protein phosphatases are assigned for phosphotyrosine residues. Interestingly, both groups of phosphatases have been shown to regulate the expression of iNOS in glial cells.
Regulation by phospho serine/threonine protein phosphatase
The hypothesis that cellular regulation of signaling pathways may utilize Ser/Thr phosphatases to modulate the phosphorylation state of critical phosphoproteins associated with the induction of iNOS was tested in rat primary astrocytes. Compounds (calyculin A, microcystin, okadaic acid, and cantharidin) that inhibit PP 1 and PP 2A were found to stimulate the LPS- and cytokine-mediated expression of iNOS and production of NO in rat primary astrocytes and C6 glial cells, suggesting that protein phosphatases may negatively regulate astroglial expression of iNOS (109). Because the activation of NF-κB is necessary for the induction of iNOS, the effect of okadaic acid on the LPS-mediated activation of NF-κB was tested in rat primary astrocytes. Interestingly, okadaic acid stimulated the LPS-mediated DNA binding as well as transcriptional activity of NF-κB, suggesting that the stimulation of iNOS expression in astrocytes by inhibitors of PP1/2A is possibly due to a stimulation of NF-κB activation. In contrast, regulation of iNOS by PP1/2A in microglia has not been reported. However, inhibition of PP1/2A has been shown to stimulate the activation of NF-κB and attenuate the expression of iNOS in rat macrophages (109), suggesting that activation of NF-κB is not sufficient for the induction of iNOS in macrophages, and that apart from NF-κB some other signaling pathway(s) sensitive to PP 1/2A is/are possibly involved in the regulation of iNOS in macrophages. Given the proximity of macrophages to brain resident microglia, it is possible that PP1/2A may regulate microglial expression of iNOS in a way similar to macrophages.
Regulation by phosphotyrosine protein phosphatase
Consistent with the regulation of iNOS by tyrosine kinases (see above), it has been found that tyrosine phosphatase(s) also play an important role in controlling the expression of iNOS. SH2 domain-containing tyrosine phosphatases (SHP)-1 and SHP-2 have two SH2 domains at the N terminus and a phosphatase catalytic domain at the C terminus capable of dephosphorylating tyrosine phosphorylated JAKs, receptors, or other cellular proteins (121, 150). Therefore, SHP-1 and SHP-2 play important roles in the control of proinflammatory cytokine signaling. Kim et al. (69) have shown that curcumin, a major component of Indian spice turmeric, inhibits the expression of iNOS in ganglioside-, LPS-, or IFN-γ-stimulated microglia via activation of tyrosine phosphatases. In fact, curcumin activates SHP-2 by tyrosine phosphorylation, and activated SHP-2 then associates with JAK1/2 and dephosphorylates these kinases to suppress the JAK–STAT pathway (Fig. 1). A previous study, showing greater iNOS expression in SHP-1 deficient astrocytes, also supports the suggested role of SHPs in regulating iNOS (90). Activation of tyrosine phosphatases that do not contain SH2 domain, like CD45, also leads to the inhibition of iNOS in microglia. For example, Tan et al. (134) showed that activation of microglial CD45 by anti-CD45 antibody cross-linking inhibits the activation of p44/42 MAPK and thereby attenuates fibrillar amyloid-β peptide-induced microglial nitric oxide production.
Transcriptional regulation by ligands of nuclear hormone receptors
Nuclear receptors (NR) are evolutionary conserved lipophilic ligand-regulated transcription factors that control gene expression. NR ligands (NRL) recruit coactivators to the DNA-bound NR, thereby transactivating target genes. Interestingly, this general modus operandi is ignored during regulation of iNOS. Several observations have now revealed that NRLs repress iNOS transcription independent of nuclear receptor itself. For example, gemfibrozil, a ligand for peroxisome proliferator-activated receptor-alpha (PPAR-α), inhibits cytokine-induced iNOS expression in human astrocytes independent of PPAR-α (102). We have found that gemfibrozil induces peroxisome proliferator-responsive element (PPRE)-dependent luciferase activity, which is inhibited by the expression of ΔPPAR-α, the dominant–negative mutant of human PPAR-α. However, ΔPPAR-α is unable to abrogate gemfibrozil-mediated inhibition of iNOS, suggesting that gemfibrozil inhibits iNOS independent of PPAR-alpha. Along similar lines, 15-deoxy-12, 14-PGJ2 (15d-PGJ2), a ligand for PPAR-α, attenuates (LPS + IFN-γ)-induced expression of iNOS in rat primary astrocytes independent of the PPAR-α itself (50). Similar observations involving downregulation of iNOS have also been recorded for rat microglia (10). Recently, ligands for other NR such as RAR and RXR have been also shown to suppress the expression of iNOS independent of NR (122). However, like PPARs, there is not enough evidence to emphasize on specific roles of RAR/RXR and their ligands separately in this case.
How may NRLs repress iNOS without actually involving the NR? Although details are yet sketchy, still we have gathered few pieces of the puzzle in the past few years to generate a broad based answer. Gemfibrozil, the PPAR-α ligand, strongly inhibited (IL-1β + IFN-γ)-induced activation of NF-κB, AP-1, and C/EBPβ but not that of STAT–GAS in human astroglial cells (102). Further probing with the NF-κB pathway in rat glial cells revealed that 15d-PGJ2, the PPAR-γ ligand, inhibits this pathway at multiple points (50). Blocking of NF-κB and other TFs is indeed a handy mode of shutting down stimulus-induced response in a short period of time. Additionally, few mechanisms have been offered to explain the blocking effect of NRLs on proinflammatory TFs. As revealed in Fig. 3, PPAR-γ ligands 15d-PGJ2 and rosiglitazone, reduce phosphorylation of the JAK–STAT pathway in activated rat astroglia and microglia, thereby leading to the suppression of JAK–STAT-dependent inflammatory responses (113). This blockage is not contingent on PPAR-γ and is mediated by rapid transcription of suppressor of cytokine signaling (SOCS) 1 and 3 (Fig. 3). Additionally, SHP-2 is also involved in the antiinflammatory action of NRLs. NRL treatment was shown to phosphorylate SHP2 within minutes (113). As phosphorylated SHP2 dephosphorylates JAK, this creates yet another avenue of blocking the JAK–STAT pathway.
Thus, we find that NRLs block iNOS and other proinflammatory genes primarily by hindering activation and subsequent recruitment of several TFs to target promoters. However, the exact mechanism(s) of blocking all these TFs remains to be elucidated.
Post-transcriptional regulation of iNOS
Although most of the studies delineate transcriptional control of the iNOS gene in glial cells, there is increasing evidence that posttranscriptional modifications may also play an important role in the regulation of iNOS in glial cells. For example, Stratman et al. (113) have shown that ibuprofen, a nonsteroidal antiinflammatory drug, inhibits the expression of iNOS protein in rat cerebellar granule cells by inhibiting the post-transcriptional processing of iNOS. According to Barger et al., dehydroepiandrosterone (DHEA), a multifunctional steroid hormone known to be involved in a variety of functional activities in the CNS, inhibits LPS-induced expression of iNOS protein in rat primary microglia via a post-transcriptional mechanism (9). However, Wang et al. have demonstrated that DHEA suppressed LPS-induced iNOS mRNA expression in BV-2 microglial cells without de novo protein synthesis and did not effect the stability of LPS-induced iNOS mRNA (141). The basis of these differences of different reports can be explained by the fact that DHEA may exhibit differential effects depending on cell types. Jeohn et al. have also shown that Go6976, an indolocarbazole compound, inhibits LPS-induced expression of iNOS in microglia at the post-transcriptional level through the destabilization of iNOS mRNA (66). Transcriptional control of iNOS requires the involvement of different transcription factors that are also involved in the transcription of many other apoptotic and antiapoptotic genes in glia and other CNS cells as well. In that context, posttranscriptional regulation of iNOS may be an important step to control aberrant induction of iNOS in glial cells.
Translational regulation of iNOS
A few studies are also present in the literature to support translational control of iNOS in glial cells. For example, in cytokine-stimulated human fetal mixed glial cultures, the iNOS mRNA was expressed within 2 h after stimulation and the iNOS protein appeared within 24 h after stimulation (34). However, the production of NO was evident at 48 h after stimulation, suggesting that posttranslational regulatory events may play an important role in iNOS-mediated NO production in human glial cells. Another classic example of iNOS translational regulation has been described recently by Lee et al. (79) in which a substrate can regulate the translation of its own enzyme mRNA. l-Arginine is the only endogenous substrate of NOS and this study shows that decreased availability of l-arginine blocked the induction of NO production and the expression of iNOS protein in cytokine-stimulated astrocytes. However, activity of iNOS promoter, induction of iNOS mRNA, and stability of iNOS protein were not inhibited under these conditions (79). This study further demonstrated that arginine depletion inhibits the translation of iNOS mRNA via negative regulation of phosphorylation status of the eukaryotic initiation factor (eIF2α), responsible for translation of iNOS mRNA (79). Because eIF2α is involved in the translation of other mRNAs as well, arginine depletion may not be a specific way to control the translation of iNOS mRNA.
Therapeutic Importance of iNOS Regulation
It is generally believed that aberrant iNOS induction in the CNS at the wrong place or at the wrong time influences the pathophysiology of several human neurodegenerative disorders, leading to detrimental consequences. Once NO is produced, it reacts with superoxide to form peroxynitrite (ONOO−), the most toxic derivative of NO. Several studies have shown that iNOS–NO–ONOO− plays an important role in neuronal loss in neurodegenerative disorders. For example, by using primary mixed cortical cultures from nNOS (–/–) and iNOS (–/minus;) mice, Adamson et al. (1) have shown iNOS-derived NO as a major mediator of HIV-1 gp41 neurotoxicity. Consistent with the observation of Xu et al. (148) that injured spinal cords express iNOS and nitrotyrosine after SCI, Isaksson et al. (61) demonstrated improved functional outcome after SCI in iNOS (–/–) mice. Along the same lines, it has been shown in a model of transient focal cerebral ischemia, antisense knockdown of iNOS mRNA reduced lesion volume by 30% (115). Many cells in the SNpc from post-mortem PD samples express considerable amounts of iNOS, whereas those from age-matched controls do not (59). Consistently, the ablation of iNOS in mutant mice significantly attenuates MPTP (Parkinsonian toxin) toxicity (32).
While the observations mentioned above suggest that NO produced from iNOS is involved in the disease process of devastating neurodegenerative disorders and inhibition of iNOS is a promising therapeutic target in these disorders, conflicting results have been reported regarding the role of NO and iNOS in experimental allergic encephalomyelitis (EAE), the animal model of MS. According to Bagasra et al. (6), the mRNA for iNOS was detectable in all brains examined from patients with MS and animals with EAE but none of the control brains. Inhibition of iNOS in both rat and mouse model of EAE by aminoguanidine also prevented the clinical development of EAE (152). Similar trends were recorded in studies with iNOS inhibitors or NO scavengers (56). Again, selective inhibition of iNOS in the CNS by intraventricular administration of antisense oligonucleotides blocks the disease process of EAE in mice (35). In contrast, the induction of EAE in iNOS (–/–) mice shows that clinical symptoms of EAE and mortality rate increase in the iNOS (–/–) mice compared to the wild-type mice (40), suggesting that the expression of iNOS may be beneficial for EAE. Therefore, at present, there are two distinct pictures regarding the involvement of iNOS in the disease process of EAE. It appears that chemical inhibitors or antisense oligonucleotides at the doses applied in vivo do not inhibit iNOS completely, therefore, partial inhibition of iNOS by chemical inhibitors of iNOS or antisense oligonucleotides against iNOS protect animals against EAE. On the other hand, complete inhibition of iNOS in iNOS (–/–) mice worsens the clinical symptoms of EAE. These speculations suggest that NO produced up to certain levels from the activation of iNOS is beneficial for EAE. It is to be noted that MS and EAE, in particular, are T cell-mediated neuroinflammatory disorders and that NO inhibits the activation of T cells and thereby may act as an immunosuppressant. However, once iNOS is activated, it produces excessive amounts of NO for an extended period of time, and NO produced in excess is cytotoxic. Therefore, partial knockdown of iNOS by pharmacological compounds to inhibit the excessive production of NO but not complete knockout of the iNOS is a feasible therapeutic approach for EAE and hence for MS.
Conclusion
The convergence of various intracellular signaling pathways to the expression of iNOS in glial cells has greatly enhanced our understanding of both the mechanism of iNOS expression and its possible control in CNS inflammatory disorders. However, the contribution and importance of any biomedical subfields should be judged by two parameters: academic and therapeutic. From the academic point of view, it is important to create a dictionary of the regulation of glial iNOS that should help in intellectual expansion of this or another subfield. For example, one can predict a possible similarity with and/or merger to another subfield that might provide a more coherent approach for better understanding of signal transduction processes in the CNS. On the other hand, from the therapeutic point of view, one might expect direct application of nontoxic drugs capable of blocking these signaling pathways for glial expression of iNOS in NO-mediated neuroinflammatory and neurodegenerative diseases. From this aspect, it has, as yet, received mixed success. The reason behind this lies in the fact that same inflammatory signaling pathways and transcription factors are utilized for the transcription of various proinflammatory molecules. Several classes of drugs that attenuate the expression of iNOS in other cells including glial cells such as statins and other mevalonate inhibitors, salicylates, nonsteroidal antiinflammatory drugs, ligands of PPAR, antioxidants, cAMP phosphodiesterase inhibitors, proteosome inhibitors, and natural compounds (curcumin) are being considered for various neurodegenerative disorders. However, in addition to suppressing the expression of iNOS, these drugs also inhibit the expression of other proinflammatory molecules, suggesting that therapeutic efficacy of these drugs cannot be attributed only to the suppression of iNOS. Therefore, the challenge is to discover signaling pathways and transcription factors specific for the activation of iNOS promoter which may not be even possible. However, analysis of the huge 5′-flanking region of the human iNOS gene (>30 kb) may be a viable option in moving forward.
Although this review compiles evidence based on the regulation of iNOS in glial cells, so far very little is known about glial cell-specific signaling pathways for the induction of iNOS. It could be an important avenue to investigate if we consider the pathologies of certain neurodegenerative disorders in which the expression of iNOS is confined mainly within CNS cells. Under these conditions, specific targeting of glial iNOS is the best option to get better therapeutic output.
Acknowledgments
This study was supported by grants from the National Institutes of Health (NS39940), National Multiple Sclerosis Society (RG3422A1/1) and Michael J. Fox Foundation for Parkinson Research.
Abbreviations
- AD
Alzheimer's disease
- ALS
amyotrophic lateral sclerosis
- APP
amyloid precursor protein
- C/EBP
CCAAT/enhancer-binding protein
- cAMP
cyclic adenosine monophosphate
- CBP
CREB binding protein
- CNS
central nervous system
- eNOS
endothelial nitric oxide synthase
- HAD
HIV-associated dementia
- HD
Huntington's disease
- HIV
human Immunodeficiency virus
- IFN
interferon
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- NO
nitric oxide
- IRF
interferon regulatory factor
- ISRE
interferon stimulated responsive factor
- LPS
lipopolysaccharide
- MBP
myelin basic protein
- MCSF
macrophage colony stimulating factor
- MPTP
1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine
- MS
multiple sclerosis
- NF-κB
nuclear factor kappaB
- nNOS
neuronal nitric oxide synthase
- NSMase
neutral sphingomyelinase
- PD
Parkinson's disease
- PPAR
peroxisome proliferators activated receptor
- PPRE
peroxisome proliferator responsive element
- ROS
reactive oxygen species
- SHP
SH2 domain containing tyrosine phosphatase
- SOCS
suppressor of cytokine signaling
- TLR
Toll-like receptor
- VCAM
vascular cell adhesion molecule
- VLA
very late antigen
References
- 1.Adamson DC, Kopnisky KL, Dawson TM, Dawson VL. Mechanisms and structural determinants of HIV-1 coat protein, gp41-induced neurotoxicity. J Neurosci. 1999;19:64–71. doi: 10.1523/JNEUROSCI.19-01-00064.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamson DC, Wildemann B, Sasaki M, Glass JD, McArthur JC, Christov VI, Dawson TM, Dawson VL. Immunologic NO synthase: elevation in severe AIDS dementia and induction by HIV-1 gp41. Science. 1996;274:1917–1921. doi: 10.1126/science.274.5294.1917. [DOI] [PubMed] [Google Scholar]
- 3.Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol. 2004;22:503–529. doi: 10.1146/annurev.immunol.22.091003.090312. [DOI] [PubMed] [Google Scholar]
- 4.Auch CJ, Saha RN, Sheikh FG, Liu X, Jacobs BL, Pahan K. Role of protein kinase R in double-stranded RNA-induced expression of nitric oxide synthase in human astroglia. FEBS Lett. 2004;563:223–228. doi: 10.1016/S0014-5793(04)00302-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayasolla K, Khan M, Singh AK, Singh I. Inflammatory mediator and beta-amyloid (25–35)-induced ceramide generation and iNOS expression are inhibited by vitamin E. Free Radic Biol Med. 2004;37:325–338. doi: 10.1016/j.freeradbiomed.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Bagasra O, Michaels FH, Zheng YM, Bobroski LE, Spitsin SV, Fu ZF, Tawadros R, Koprowski H. Activation of the inducible form of nitric oxide synthase in the brains of patients with multiple sclerosis. Proc Natl Acad Sci USA. 1995;92:12041–12045. doi: 10.1073/pnas.92.26.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbeito LH, Pehar M, Cassina P, Vargas MR, Peluffo H, Viera L, Estevez AG, Beckman JS. A role for astrocytes in motor neuron loss in amyotrophic lateral sclerosis. Brain Res Brain Res Rev. 2004;47:263–274. doi: 10.1016/j.brainresrev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Barger SW, Harmon AD. Microglial activation by Alzheimer amyloid precursor protein and modulation by apolipoprotein E. Nature. 1997;388:878–881. doi: 10.1038/42257. [DOI] [PubMed] [Google Scholar]
- 9.Barger SW, Chavis JA, Drew PD. Dehydroepiandrosterone inhibits microglial nitric oxide production in a stimulus-specific manner. J Neurosci Res. 2000;62:503–509. doi: 10.1002/1097-4547(20001115)62:4<503::AID-JNR4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 10.Bernardo A, Levi G, Minghetti L. Role of the peroxisome proliferator-activated receptor-gamma (PPAR-gamma) and its natural ligand 15-deoxy-Delta12, 14-prostaglandin J2 in the regulation of microglial functions. Eur J Neurosci. 2000;12:2215–2223. doi: 10.1046/j.1460-9568.2000.00110.x. [DOI] [PubMed] [Google Scholar]
- 11.Bethea JR, Castro M, Keane RW, Lee TT, Dietrich WD, Yezierski RP. Traumatic spinal cord injury induces nuclear factor-kappaB activation. J Neurosci. 1998;18:3251–3260. doi: 10.1523/JNEUROSCI.18-09-03251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhat NR, Fan F. Adenovirus infection induces microglial activation: involvement of mitogen-activated protein kinase pathways. Brain Res. 2002;948:93–101. doi: 10.1016/s0006-8993(02)02953-0. [DOI] [PubMed] [Google Scholar]
- 13.Bhat NR, Feinstein DL, Shen Q, Bhat AN. p38 MAPK-mediated transcriptional activation of inducible nitric-oxide synthase in glial cells. Roles of nuclear factors, nuclear factor kappa B, cAMP response element-binding protein, CCAAT/enhancer-binding protein-beta, and activating transcription factor-2. J Biol Chem. 2002;277:29584–9592. doi: 10.1074/jbc.M204994200. [DOI] [PubMed] [Google Scholar]
- 14.Bhat NR, Shen Q, Fan F. TAK1-mediated induction of nitric oxide synthase gene expression in glial cells. J Neurochem. 2003;87:238–247. doi: 10.1046/j.1471-4159.2003.01998.x. [DOI] [PubMed] [Google Scholar]
- 15.Bhat NR, Zhang P, Lee JC, Hogan EL. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci. 1998;18:1633–1641. doi: 10.1523/JNEUROSCI.18-05-01633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bodles AM, Barger SW. Secreted beta-amyloid precursor protein activates microglia via JNK and p38-MAPK. Neurobiol Aging. 2005;26:9–16. doi: 10.1016/j.neurobiolaging.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 17.Boje KM. Nitric oxide neurotoxicity in neurodegenerative diseases. Front Biosci. 2004;9:763–776. doi: 10.2741/1268. [DOI] [PubMed] [Google Scholar]
- 18.Bowman CC, Rasley A, Tranguch SL, Marriott I. Cultured astrocytes express toll-like receptors for bacterial products. Glia. 2003;43:281–291. doi: 10.1002/glia.10256. [DOI] [PubMed] [Google Scholar]
- 19.Brodie C, Weizman N, Katzoff A, Lustig S, Kobiler D. Astrocyte activation by Sindbis virus: expression of GFAP, cytokines, and adhesion molecules. Glia. 1997;19:275–285. [PubMed] [Google Scholar]
- 20.Cantrell DA. Phosphoinositide 3-kinase signalling pathways. J Cell Sci. 2001;114:1439–1445. doi: 10.1242/jcs.114.8.1439. [DOI] [PubMed] [Google Scholar]
- 21.Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol. 1992;149:2736–2741. [PubMed] [Google Scholar]
- 22.Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, Hobbs W, Vonsattel JP, Cha JH, Friedlander RM. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med. 2000;6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- 23.Chu SC, Marks–Konczalik J, Wu HP, Banks TC, Moss J. Analysis of the cytokine-stimulated human inducible nitric oxide synthase (iNOS) gene: characterization of differences between human and mouse iNOS promoters. Biochem Biophys Res Commun. 1998;248:871–878. doi: 10.1006/bbrc.1998.9062. [DOI] [PubMed] [Google Scholar]
- 24.Combs CK, Karlo JC, Kao SC, Landreth GE. beta-Amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J Neurosci. 2001;21:1179–1188. doi: 10.1523/JNEUROSCI.21-04-01179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cordle A, Landreth G. 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors attenuate beta-amyloid-induced microglial inflammatory responses. J Neurosci. 2005;25:299–307. doi: 10.1523/JNEUROSCI.2544-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cvetkovic I, Miljkovic D, Vuckovic O, Harhaji L, Nikolic Z, Trajkovic V, Mostarica Stojkovic M. Taxol activates inducible nitric oxide synthase in rat astrocytes: the role of MAP kinases and NF-kappaB. Cell Mol Life Sci. 2004;61:1167–1175. doi: 10.1007/s00018-004-3408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalpke AH, Schafer MK, Frey M, Zimmermann S, Tebbe J, Weihe E, Heeg K. Immunostimulatory CpG-DNA activates murine microglia. J Immunol. 2002;168:4854–4863. doi: 10.4049/jimmunol.168.10.4854. [DOI] [PubMed] [Google Scholar]
- 28.Dasgupta S, Jana M, Liu X, Pahan K. Myelin basic protein-primed T cells induce nitric oxide synthase in microglial cells. Implications for multiple sclerosis. J Biol Chem. 2002;277:39327–39333. doi: 10.1074/jbc.M111841200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dasgupta S, Jana M, Zhou Y, Fung YK, Ghosh S, Pahan K. Antineuroinflammatory effect of NF-kappaB essential modifier-binding domain peptides in the adoptive transfer model of experimental allergic encephalomyelitis. J Immunol. 2004;173:1344–1354. doi: 10.4049/jimmunol.173.2.1344. [DOI] [PubMed] [Google Scholar]
- 30.Dawson VL, Dawson TM. Nitric oxide in neurodegeneration. Prog Brain Res. 1998;118:215–229. doi: 10.1016/s0079-6123(08)63210-0. [DOI] [PubMed] [Google Scholar]
- 31.de Vera ME, Shapiro RA, Nussler AK, Mudgett JS, Simmons RL, Morris SM, Jr, Billiar TR, Geller DA. Transcriptional regulation of human inducible nitric oxide synthase (NOS2) gene by cytokines: initial analysis of the human NOS2 promoter. Proc Natl Acad Sci USA. 1996;93:1054–1059. doi: 10.1073/pnas.93.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dehmer T, Lindenau J, Haid S, Dichgans J, Schulz JB. Deficiency of inducible nitric oxide synthase protects against MPTP toxicity in vivo. J Neurochem. 2000;74:2213–2216. doi: 10.1046/j.1471-4159.2000.0742213.x. [DOI] [PubMed] [Google Scholar]
- 33.Dell'Albani P, Santangelo R, Torrisi L, Nicoletti VG, de Vellis J, Giuffrida Stella AM. JAK/STAT signaling pathway mediates cytokine-induced iNOS expression in primary astroglial cell cultures. J Neurosci Res. 2001;65:417–424. doi: 10.1002/jnr.1169. [DOI] [PubMed] [Google Scholar]
- 34.Ding M, St Pierre BA, Parkinson JF, Medberry P, Wong JL, Rogers NE, Ignarro LJ, Merrill JE. Inducible nitric-oxide synthase and nitric oxide production in human fetal astrocytes and microglia. A kinetic analysis. J Biol Chem. 1997;272:11327–11335. doi: 10.1074/jbc.272.17.11327. [DOI] [PubMed] [Google Scholar]
- 35.Ding M, Zhang M, Wong JL, Rogers NE, Ignarro LJ, Voskuhl RR. Antisense knockdown of inducible nitric oxide synthase inhibits induction of experimental autoimmune encephalomyelitis in SJL/J mice. J Immunol. 1998;160:2560–2564. [PubMed] [Google Scholar]
- 36.Dlaska M, Weiss G. Central role of transcription factor NF-IL6 for cytokine and iron-mediated regulation of murine inducible nitric oxide synthase expression. J Immunol. 1999;162:6171–6177. [PubMed] [Google Scholar]
- 37.Eberhardt W, Pluss C, Hummel R, Pfeilschifter J. Molecular mechanisms of inducible nitric oxide synthase gene expression by IL-1beta and cAMP in rat mesangial cells. J Immunol. 1998;160:4961–4969. [PubMed] [Google Scholar]
- 38.Fabrizi C, Silei V, Menegazzi M, Salmona M, Bugiani O, Tagliavini F, Suzuki H, Lauro GM. The stimulation of inducible nitric-oxide synthase by the prion protein fragment 106–126 in human microglia is tumor necrosis factor-alpha-dependent and involves p38 mitogen-activated protein kinase. J Biol Chem. 2001;276:25692–25696. doi: 10.1074/jbc.M100133200. [DOI] [PubMed] [Google Scholar]
- 39.Feinstein DL, Galea E, Reis DJ. Suppression of glial iNOS expression by tyrosine kinase inhibitors. Ann NY Acad Sci. 1994;738:325–328. doi: 10.1111/j.1749-6632.1994.tb21818.x. [DOI] [PubMed] [Google Scholar]
- 40.Fenyk–Melody JE, Garrison AE, Brunnert SR, Weidner JR, Shen F, Shelton BA, Mudgett JS. Experimental autoimmune encephalomyelitis is exacerbated in mice lacking the NOS2 gene. J Immunol. 1998;160:2940–2946. [PubMed] [Google Scholar]
- 41.Fiebich BL, Butcher RD, Gebicke–Haerter PJ. Protein kinase C-mediated regulation of inducible nitric oxide synthase expression in cultured microglial cells. J Neuroimmunol. 1998;92:170–178. doi: 10.1016/s0165-5728(98)00201-x. [DOI] [PubMed] [Google Scholar]
- 42.Fiebich BL, Lieb K, Engels S, Heinrich M. Inhibition of LPS-induced p42/44 MAP kinase activation and iNOS/NO synthesis by parthenolide in rat primary microglial cells. J Neuroimmunol. 2002;132:18–24. doi: 10.1016/s0165-5728(02)00279-5. [DOI] [PubMed] [Google Scholar]
- 43.Fujimura M, Tominaga T, Kato I, Takasawa S, Kawase M, Taniguchi T, Okamoto H, Yoshimoto T. Attenuation of nitric oxide synthase induction in IRF-1-deficient glial cells. Brain Res. 1997;759:247–250. doi: 10.1016/s0006-8993(97)00264-3. [DOI] [PubMed] [Google Scholar]
- 44.Galea E, Feinstein DL, Reis DJ. Induction of calcium-independent nitric oxide synthase activity in primary rat glial cultures. Proc Natl Acad Sci USA. 1992;89:10945–10949. doi: 10.1073/pnas.89.22.10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galea E, Reis DJ, Feinstein DL. Cloning and expression of inducible nitric oxide synthase from rat astrocytes. J Neurosci Res. 1994;37:406–414. doi: 10.1002/jnr.490370313. [DOI] [PubMed] [Google Scholar]
- 46.Galea E, Reis DJ, Fox ES, Xu H, Feinstein DL. CD14 mediate endotoxin induction of nitric oxide synthase in cultured brain glial cells. J Neuroimmunol. 1996;64:19–28. doi: 10.1016/0165-5728(95)00143-3. [DOI] [PubMed] [Google Scholar]
- 47.Ganster RW, Taylor BS, Shao L, Geller DA. Complex regulation of human inducible nitric oxide synthase gene transcription by Stat 1 and NF-kappa B. Proc Natl Acad Sci USA. 2001;98:8638–8643. doi: 10.1073/pnas.151239498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao J, Morrison DC, Parmely TJ, Russell SW, Murphy WJ. An interferon-gamma-activated site (GAS) is necessary for full expression of the mouse iNOS gene in response to interferon-gamma and lipopolysaccharide. J Biol Chem. 1997;272:1226–1230. doi: 10.1074/jbc.272.2.1226. [DOI] [PubMed] [Google Scholar]
- 49.Giri S, Jatana M, Rattan R, Won JS, Singh I, Singh AK. Galactosylsphingosine (psychosine)-induced expression of cytokine-mediated inducible nitric oxide synthases via AP-1 and C/EBP: implications for Krabbe disease. FASEB J. 2002;16:661–672. doi: 10.1096/fj.01-0798com. [DOI] [PubMed] [Google Scholar]
- 50.Giri S, Rattan R, Singh AK, Singh I. The 15-deoxy-delta12,14-prostaglandin J2 inhibits the inflammatory response in primary rat astrocytes via down-regulating multiple steps in phosphatidylinositol 3-kinase-Akt-NF-kappaB-p300 pathway independent of peroxisome proliferator-activated receptor gamma. J Immunol. 2004;173:5196–5208. doi: 10.4049/jimmunol.173.8.5196. [DOI] [PubMed] [Google Scholar]
- 51.Goldring CE, Reveneau S, Algarte M, Jeannin JF. In vivo footprinting of the mouse inducible nitric oxide synthase gene: inducible protein occupation of numerous sites including Oct and NF-IL6. Nucleic Acids Res. 1996;24:1682–1687. doi: 10.1093/nar/24.9.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 53.Gomez RM, Yep A, Schattner M, Berria MI. Junin virus-induced astrocytosis is impaired by iNOS inhibition. J Med Virol. 2003;69:145–149. doi: 10.1002/jmv.10254. [DOI] [PubMed] [Google Scholar]
- 54.Graves LM, Bornfeldt KE, Raines EW, Potts BC, Macdonald SG, Ross R, Krebs EG. Protein kinase A antagonizes platelet-derived growth factor-induced signaling by mitogen-activated protein kinase in human arterial smooth muscle cells. Proc Natl Acad Sci USA. 1993;90:10300–10304. doi: 10.1073/pnas.90.21.10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hannun YA. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994;269:3125–3128. [PubMed] [Google Scholar]
- 56.Hooper DC, Bagasra O, Marini JC, Zborek A, Ohnishi ST, Kean R, Champion JM, Sarker AB, Bobroski L, Farber JL, Akaike T, Maeda H, Koprowski H. Prevention of experimental allergic encephalomyelitis by targeting nitric oxide and peroxynitrite: implications for the treatment of multiple sclerosis. Proc Natl Acad Sci USA. 1997;94:2528–2533. doi: 10.1073/pnas.94.6.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hosoi T, Suzuki S, Nomura J, Ono A, Okuma Y, Akira S, Nomura Y, Brodie C, Weizman N, Katzoff A, Lustig S, Kobiler D. Bacterial DNA induced iNOS expression through MyD88-p38 MAP kinase in mouse primary cultured glial cells. Brain Res Mol Brain Res. 2004;124:159–164. doi: 10.1016/j.molbrainres.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 58.Hua LL, Zhao ML, Cosenza M, Kim MO, Huang H, Tanowitz HB, Brosnan CF, Lee SC. Role of mitogen-activated protein kinases in inducible nitric oxide synthase and TNFalpha expression in human fetal astrocytes. J Neuroimmunol. 2002;126:180–189. doi: 10.1016/s0165-5728(02)00055-3. [DOI] [PubMed] [Google Scholar]
- 59.Hunot S, Boissiere F, Faucheux B, Brugg B, Mouatt–Prigent A, Agid Y, Hirsch EC. Nitric oxide synthase and neuronal vulnerability in Parkinson's disease. Neuroscience. 1996;72:355–363. doi: 10.1016/0306-4522(95)00578-1. [DOI] [PubMed] [Google Scholar]
- 60.Ikeda U, Yamamoto K, Ichida M, Ohkawa F, Murata M, Iimura O, Kusano E, Asano Y, Shimada K. Cyclic AMP augments cytokine-stimulated nitric oxide synthesis in rat cardiac myocytes. J Mol Cell Cardiol. 1996;28:789–95. doi: 10.1006/jmcc.1996.0073. [DOI] [PubMed] [Google Scholar]
- 61.Isaksson J, Farooque M, Olsson Y. Improved functional outcome after spinal cord injury in iNOS-deficient mice. Spinal Cord. 2005;43:167–170. doi: 10.1038/sj.sc.3101672. [DOI] [PubMed] [Google Scholar]
- 62.Jaffrey SR, Snyder SH. Nitric oxide: a neural messenger. Annu Rev Cell Dev Biol. 1995;11:417–440. doi: 10.1146/annurev.cb.11.110195.002221. [DOI] [PubMed] [Google Scholar]
- 63.Jana M, Anderson JA, Saha RN, Liu X, Pahan K. Regulation of inducible nitric oxide synthase in proinflammatory cytokine-stimulated human primary astrocytes. Free Radic Biol Med. 2005;38:655–664. doi: 10.1016/j.freeradbiomed.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 64.Jana M, Liu X, Koka S, Ghosh S, Petro TM, Pahan K. Ligation of CD40 stimulates the induction of nitric-oxide synthase in microglial cells. J Biol Chem. 2001;276:44527–44533. doi: 10.1074/jbc.M106771200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jarosinski KW, Njaa BL, O'connell PH, Schat KA. Pro-inflammatory responses in chicken spleen and brain tissues after infection with very virulent plus Marek's disease virus. Viral Immunol. 2005;18:148–161. doi: 10.1089/vim.2005.18.148. [DOI] [PubMed] [Google Scholar]
- 66.Jeohn GH, Chang RC, Kim WG, Wilson B, Mohney RP, Wetsel WC, Hong JS. Post-transcriptional inhibition of lipopolysaccharide-induced expression of inducible nitric oxide synthase by Go6976 in murine microglia. Brain Res Mol Brain Res. 2000;79:18–31. doi: 10.1016/s0169-328x(00)00081-4. [DOI] [PubMed] [Google Scholar]
- 67.Kawase M, Kinouchi H, Kato I, Akabane A, Kondo T, Arai S, Fujimura M, Okamoto H, Yoshimoto T. Inducible nitric oxide synthase following hypoxia in rat cultured glial cells. Brain Res. 1996;738:319–322. doi: 10.1016/s0006-8993(96)00924-9. [DOI] [PubMed] [Google Scholar]
- 68.Kikuchi A, Williams LT. The post-translational modification of ras p21 is important for Raf-1 activation. J Biol Chem. 1994;269:20054–20059. [PubMed] [Google Scholar]
- 69.Kim HY, Park EJ, Joe EH, Jou I. Curcumin suppresses Janus kinase-STAT inflammatory signaling through activation of Src homology 2 domain-containing tyrosine phosphatase 2 in brain microglia. J Immunol. 2003;171:6072–6079. doi: 10.4049/jimmunol.171.11.6072. [DOI] [PubMed] [Google Scholar]
- 70.Kitamura Y, Takahashi H, Nomura Y, Taniguchi T. Possible involvement of Janus kinase Jak2 in interferon-gamma induction of nitric oxide synthase in rat glial cells. Eur J Pharmacol. 1996;306:297–306. doi: 10.1016/0014-2999(96)00212-9. [DOI] [PubMed] [Google Scholar]
- 71.Kizaki T, Suzuki K, Hitomi Y, Iwabuchi K, Onoe K, Haga S, Ishida H, Ookawara T, Suzuki K, Ohno H. Negative regulation of LPS-stimulated expression of inducible nitric oxide synthase by AP-1 in macrophage cell line J774A.1. Biochem Biophys Res Commun. 2001;289:1031–1038. doi: 10.1006/bbrc.2001.6123. [DOI] [PubMed] [Google Scholar]
- 72.Kleinert H, Euchenhofer C, Ihrig-Biedert I, Forstermann U. In murine 3T3 fibroblasts, different second messenger pathways resulting in the induction of NO synthase II (iNOS) converge in the activation of transcription factor NF-kappaB. J Biol Chem. 1996;271:6039–6044. doi: 10.1074/jbc.271.11.6039. [DOI] [PubMed] [Google Scholar]
- 73.Koide M, Kawahara Y, Tsuda T, Yokoyama M. Cytokine-induced expression of an inducible type of nitric oxide synthase gene in cultured vascular smooth muscle cells. FEBS Lett. 1993;318:213–217. doi: 10.1016/0014-5793(93)80514-u. [DOI] [PubMed] [Google Scholar]
- 74.Kolesnick R, Golde DW. The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell. 1994;77:325–328. doi: 10.1016/0092-8674(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 75.Kolyada AY, Madias NE. Transcriptional regulation of the human iNOS gene by IL-1beta in endothelial cells. Mol Med. 2001;7:329–343. [PMC free article] [PubMed] [Google Scholar]
- 76.Kong LY, McMillian MK, Maronpot R, Hong JS. Protein tyrosine kinase inhibitors suppress the production of nitric oxide in mixed glia, microglia-enriched or astrocyte-enriched cultures. Brain Res. 1996;729:102–109. [PubMed] [Google Scholar]
- 77.Kovacs KA, Steinmann M, Magistretti PJ, Halfon O, Cardinaux JR. CCAAT/enhancer-binding protein family members recruit the coactivator CREB-binding protein and trigger its phosphorylation. J Biol Chem. 2003;278:36959–36965. doi: 10.1074/jbc.M303147200. [DOI] [PubMed] [Google Scholar]
- 78.Krof AS, Marks–Konczalik J, Moss J. Mitogen-activated protein kinases mediate activator protein-1-dependent human inducible nitric-oxide synthase promoter activation. J Biol Chem. 2001;276:8445–8452. doi: 10.1074/jbc.M009563200. [DOI] [PubMed] [Google Scholar]
- 79.Lee J, Ryu H, Ferrante RJ, Morris SM, Jr, Ratan RR. Translational control of inducible nitric oxide synthase expression by arginine can explain the arginine paradox. Proc Natl Acad Sci USA. 2003;100:4843–4848. doi: 10.1073/pnas.0735876100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci USA. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lekstrom–Himes J, Xanthopoulos KG. Biological role of the CCAAT/enhancer-binding protein family of transcription factors. J Biol Chem. 1998;273:28545–28548. doi: 10.1074/jbc.273.44.28545. [DOI] [PubMed] [Google Scholar]
- 82.Leroith D, Nissley P. Knock your SOCS off! J Clin Invest. 2005;115:233–236. doi: 10.1172/JCI24228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liberatore GT, Jackson–Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- 84.Liu B, Gao HM, Wang JY, Jeohn GH, Cooper CL, Hong JS. Role of nitric oxide in inflammation-mediated neurodegeneration. Ann NY Acad Sci. 2002;962:318–331. doi: 10.1111/j.1749-6632.2002.tb04077.x. [DOI] [PubMed] [Google Scholar]
- 85.Liu X, Jana M, Dasgupta S, Koka S, He J, Wood C, Pahan K. Human immunodeficiency virus type 1 (HIV-1) tat induces nitric-oxide synthase in human astroglia. J Biol Chem. 2002;277:39312–39319. doi: 10.1074/jbc.M205107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mack CL, Vanderlugt–Castaneda CL, Neville KL, Miller SD. Microglia are activated to become competent antigen presenting and effector cells in the inflammatory environment of the Theiler's virus model of multiple sclerosis. J Neuroimmunol. 2003;144:68–79. doi: 10.1016/j.jneuroim.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 87.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 88.Marcus JS, Karackattu SL, Fleegal MA, Sumners C. Cytokine-stimulated inducible nitric oxide synthase expression in astroglia: role of Erk mitogen-activated protein kinase and NF-kappaB. Glia. 2003;41:152–160. doi: 10.1002/glia.10168. [DOI] [PubMed] [Google Scholar]
- 89.Martin E, Nathan C, Xie QW. Role of interferon regulatory factor 1 in induction of nitric oxide synthase. J Exp Med. 1994;180:977–984. doi: 10.1084/jem.180.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Massa PT, Wu C. Increased inducible activation of NF-kappaB and responsive genes in astrocytes deficient in the protein tyrosine phosphatase SHP-1. J Interferon Cytokine Res. 1998;18:499–507. doi: 10.1089/jir.1998.18.499. [DOI] [PubMed] [Google Scholar]
- 91.Min KJ, Pyo HK, Yang MS, Ji KA, Jou I, Joe EH. Gangliosides activate microglia via protein kinase C and NADPH oxidase. Glia. 2004;48:197–206. doi: 10.1002/glia.20069. [DOI] [PubMed] [Google Scholar]
- 92.Min KJ, Yang MS, Jou I, Joe EH. Protein kinase A mediates microglial activation induced by plasminogen and gangliosides. Exp Mol Med. 2004;36:461–467. doi: 10.1038/emm.2004.58. [DOI] [PubMed] [Google Scholar]
- 93.Minghetti L, Nicolini A, Polazzi E, Creminon C, Maclouf J, Levi G. Inducible nitric oxide synthase expression in activated rat microglial cultures is downregulated by exogenous prostaglandin E2 and by cyclooxygenase inhibitors. Glia. 1997;19:152–160. [PubMed] [Google Scholar]
- 94.Mitrovic B, Ignarro LJ, Montestruque S, Smoll A, Merrill JE. Nitric oxide as a potential pathological mechanism in demyelination: its differential effects on primary glial cells in vitro. Neuroscience. 1994;61:575–585. doi: 10.1016/0306-4522(94)90435-9. [DOI] [PubMed] [Google Scholar]
- 95.Muhl H, Kunz D, Pfeilschifter J. Expression of nitric oxide synthase in rat glomerular mesangial cells mediated by cyclic AMP. Br J Pharmacol. 1994;112:1–8. doi: 10.1111/j.1476-5381.1994.tb13019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Murphy GM, Jr, Yang L, Cordell B. Macrophage colony-stimulating factor augments beta-amyloid-induced interleukin-1, interleukin-6, and nitric oxide production by microglial cells. J Biol Chem. 1998;273:20967–20971. doi: 10.1074/jbc.273.33.20967. [DOI] [PubMed] [Google Scholar]
- 97.Na HJ, Lee SJ, Kang YC, Cho YL, Nam WD, Kim PK, Ha KS, Chung HT, Lee H, Kwon YG, Koh JS, Kim YM. Inhibition of farnesyltransferase prevents collagen-induced arthritis by down-regulation of inflammatory gene expression through suppression of p21(ras)-dependent NF-kappaB activation. J Immunol. 2004;173:1276–1283. doi: 10.4049/jimmunol.173.2.1276. [DOI] [PubMed] [Google Scholar]
- 98.Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 99.Nicholson TE, Dibb S, Renton KW. Nitric oxide mediates an LPS-induced depression of cytochrome P450 (CYP1A) activity in astrocytes. Brain Res. 2004;1029:148–154. doi: 10.1016/j.brainres.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 100.Nishiya T, Uehara T, Nomura Y. Herbimycin A suppresses NF-kappa B activation and tyrosine phosphorylation of JAK2 and the subsequent induction of nitric oxide synthase in C6 glioma cells. FEBS Lett. 1995;371:333–336. doi: 10.1016/0014-5793(95)00933-z. [DOI] [PubMed] [Google Scholar]
- 101.Nunokawa Y, Oikawa S, Tanaka S. Human inducible nitric oxide synthase gene is transcriptionally regulated by nuclear factor-kappaB dependent mechanism. Biochem Biophys Res Commun. 1996;223:347–352. doi: 10.1006/bbrc.1996.0897. [DOI] [PubMed] [Google Scholar]
- 102.Pahan K, Jana M, Liu X, Taylor BS, Wood C, Fischer SM. Gemfibrozil, a lipid-lowering drug, inhibits the induction of nitric-oxide synthase in human astrocytes. J Biol Chem. 2002;277:45984–45991. doi: 10.1074/jbc.M200250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pahan K, Liu X, McKinney MJ, Wood C, Sheikh FG, Raymond JR. Expression of a dominant-negative mutant of p21(ras) inhibits induction of nitric oxide synthase and activation of nuclear factor-kappaB in primary astrocytes. J Neurochem. 2000;74:2288–2295. doi: 10.1046/j.1471-4159.2000.0742288.x. [DOI] [PubMed] [Google Scholar]
- 104.Pahan K, Liu X, Wood C, Raymond JR. Expression of a constitutively active form of phosphatidylinositol 3-kinase inhibits the induction of nitric oxide synthase in human astrocytes. FEBS Lett. 2000;472:203–207. doi: 10.1016/s0014-5793(00)01465-4. [DOI] [PubMed] [Google Scholar]
- 105.Pahan K, Namboodiri AM, Sheikh FG, Smith BT, Singh I. Increasing cAMP attenuates induction of inducible nitric-oxide synthase in rat primary astrocytes. J Biol Chem. 1997;272:7786–7791. doi: 10.1074/jbc.272.12.7786. [DOI] [PubMed] [Google Scholar]
- 106.Pahan K, Raymond JR, Singh I. Inhibition of phosphatidylinositol 3-kinase induces nitric-oxide synthase in lipopolysaccharide- or cytokine-stimulated C6 glial cells. J Biol Chem. 1999;274:7528–7536. doi: 10.1074/jbc.274.11.7528. [DOI] [PubMed] [Google Scholar]
- 107.Pahan K, Sheikh FG, Khan M, Namboodiri AM, Singh I. Sphingomyelinase and ceramide stimulate the expression of inducible nitric-oxide synthase in rat primary astrocytes. J Biol Chem. 1998;273:2591–2600. doi: 10.1074/jbc.273.5.2591. [DOI] [PubMed] [Google Scholar]
- 108.Pahan K, Sheikh FG, Liu X, Hilger S, McKinney M, Petro TM. Induction of nitric-oxide synthase and activation of NF-kappaB by interleukin-12 p40 in microglial cells. J Biol Chem. 2001;276:7899–7905. doi: 10.1074/jbc.M008262200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pahan K, Sheikh FG, Namboodiri AM, Singh I. Inhibitors of protein phosphatase 1 and 2A differentially regulate the expression of inducible nitric-oxide synthase in rat astrocytes and macrophages. J Biol Chem. 1998;273:12219–12226. doi: 10.1074/jbc.273.20.12219. [DOI] [PubMed] [Google Scholar]
- 110.Pahan K, Sheikh FG, Namboodiri AM, Singh I. Lovastatin and phenylacetate inhibit the induction of nitric oxide synthase and cytokines in rat primary astrocytes, microglia, and macrophages. J Clin Invest. 1997;100:2671–2679. doi: 10.1172/JCI119812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pahan K, Sheikh FG, Namboodiri AM, Singh I. N-acetyl cysteine inhibits induction of no production by endotoxin or cytokine stimulated rat peritoneal macrophages, C6 glial cells and astrocytes. Free Radic Biol Med. 1998;24:39–48. doi: 10.1016/s0891-5849(97)00137-8. [DOI] [PubMed] [Google Scholar]
- 112.Pannu R, Won JS, Khan M, Singh AK, Singh I. A novel role of lactosylceramide in the regulation of lipopolysaccharide/interferon-gamma-mediated inducible nitric oxide synthase gene expression: implications for neuroinflammatory diseases. J Neurosci. 2004;24:5942–5954. doi: 10.1523/JNEUROSCI.1271-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Park EJ, Park SY, Joe EH, Jou I. 15d-PGJ2 and rosiglitazone suppress Janus kinase-STAT inflammatory signaling through induction of suppressor of cytokine signaling 1 (SOCS1) and SOCS3 in glia. J Biol Chem. 2003;278:14747–14752. doi: 10.1074/jbc.M210819200. [DOI] [PubMed] [Google Scholar]
- 114.Park SY, Lee H, Hur J, Kim SY, Kim H, Park JH, Cha S, Kang SS, Cho GJ, Choi WS, Suk K. Hypoxia induces nitric oxide production in mouse microglia via p38 mitogen-activated protein kinase pathway. Brain Res Mol Brain Res. 2002;107:9–16. doi: 10.1016/s0169-328x(02)00421-7. [DOI] [PubMed] [Google Scholar]
- 115.Parmentier–Batteur S, Bohme GA, Lerouet D, Zhou–Ding L, Beray V, Margaill I, Plotkine M. Antisense oligodeoxynucleotide to inducible nitric oxide synthase protects against transient focal cerebral ischemia-induced brain injury. J Cereb Blood Flow Metab. 2001;21:15–21. doi: 10.1097/00004647-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 116.Pawate S, Shen Q, Fan F, Bhat NR. Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J Neurosci Res. 2004;77:540–551. doi: 10.1002/jnr.20180. [DOI] [PubMed] [Google Scholar]
- 117.Petrova TV, Akama KT, Van Eldik LJ. Cyclopentenone prostaglandins suppress activation of microglia: down-regulation of inducible nitric-oxide synthase by 15-deoxy-Delta12,14-prostaglandin J2. Proc Natl Acad Sci USA. 1999;96:4668–4673. doi: 10.1073/pnas.96.8.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Polazzi E, Levi G, Minghetti L. Human immunodeficiency virus type 1 Tat protein stimulates inducible nitric oxide synthase expression and nitric oxide production in microglial cultures. J Neuropathol Exp Neurol. 1999;58:825–831. doi: 10.1097/00005072-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 119.Possel H, Noack H, Putzke J, Wolf G, Sies H. Selective upregulation of inducible nitric oxide synthase (iNOS) by lipopolysaccharide (LPS) and cytokines in microglia: in vitro and in vivo studies. Glia. 2000;32:51–59. doi: 10.1002/1098-1136(200010)32:1<51::aid-glia50>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 120.Pyo H, Jou I, Jung S, Joe E. cAMP potentiates beta-amyloid-induced nitric oxide release from microglia. Neuroreport. 1999;10:37–40. doi: 10.1097/00001756-199901180-00007. [DOI] [PubMed] [Google Scholar]
- 121.Qu CK. Role of the SHP-2 tyrosine phosphatase in cytokine-induced signaling and cellular response. Biochim Biophys Acta. 2002;1592:297–301. doi: 10.1016/s0167-4889(02)00322-1. [DOI] [PubMed] [Google Scholar]
- 122.Royal W, 3rd, Leander M, Chen YE, Major EO, Bissonnette RP. Nuclear receptor activation and interaction with morphine. J Neuroimmunol. 2004;157:61–65. doi: 10.1016/j.jneuroim.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 123.Ryu JK, Kim SU, McLarnon JG. Blockade of quinolinic acid-induced neurotoxicity by pyruvate is associated with inhibition of glial activation in a model of Huntington's disease. Exp Neurol. 2004;187:150–159. doi: 10.1016/j.expneurol.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 124.Saura M, Zaragoza C, Bao C, McMillan A, Lowenstein CJ. Interaction of interferon regulatory factor-1 and nuclear factor kappaB during activation of inducible nitric oxide synthase transcription. J Mol Biol. 1999;289:459–471. doi: 10.1006/jmbi.1999.2752. [DOI] [PubMed] [Google Scholar]
- 125.Schoneboom BA, Catlin KM, Marty AM, Grieder FB. Inflammation is a component of neurodegeneration in response to Venezuelan equine encephalitis virus infection in mice. J Neuroimmunol. 2000;109:132–146. doi: 10.1016/s0165-5728(00)00290-3. [DOI] [PubMed] [Google Scholar]
- 126.Schwartz C, Beck K, Mink S, Schmolke M, Budde B, Wenning D, Klempnauer KH. Recruitment of p300 by C/EBPbeta triggers phosphorylation of p300 and modulates coactivator activity. EMBO J. 2003;22:882–892. doi: 10.1093/emboj/cdg076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Simmons ML, Murphy S. Induction of nitric oxide synthase in glial cells. J Neurochem. 1992;59:897–905. doi: 10.1111/j.1471-4159.1992.tb08328.x. [DOI] [PubMed] [Google Scholar]
- 128.Simmons ML, Murphy S. Roles for protein kinases in the induction of nitric oxide synthase in astrocytes. Glia. 1994;11:227–234. doi: 10.1002/glia.440110303. [DOI] [PubMed] [Google Scholar]
- 129.Sola C, Casal C, Tusell JM, Serratosa J. Astrocytes enhance lipopolysaccharide-induced nitric oxide production by microglial cells. Eur J Neurosci. 2002;16:1275–1283. doi: 10.1046/j.1460-9568.2002.02199.x. [DOI] [PubMed] [Google Scholar]
- 130.Sowa G, Przewlocki R. cAMP analogues and cholera toxin stimulate the accumulation of nitrite in rat peritoneal macrophage cultures. Eur J Pharmacol. 1994;266:125–129. doi: 10.1016/0922-4106(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 131.Spink J, Evans T. Binding of the transcription factor interferon regulatory factor-1 to the inducible nitric-oxide synthase promoter. J Biol Chem. 1997;272:24417–24425. doi: 10.1074/jbc.272.39.24417. [DOI] [PubMed] [Google Scholar]
- 132.Spink J, Cohen J, Evans TJ. The cytokine responsive vascular smooth muscle cell enhancer of inducible nitric oxide synthase. Activation by nuclear factor-kappa B. J Biol Chem. 1995;270:29541–29547. doi: 10.1074/jbc.270.49.29541. [DOI] [PubMed] [Google Scholar]
- 133.Stratman NC, Carter DB, Sethy VH. Ibuprofen: effect on inducible nitric oxide synthase. Brain Res Mol Brain Res. 1997;50:107–112. doi: 10.1016/s0169-328x(97)00168-x. [DOI] [PubMed] [Google Scholar]
- 134.Tan J, Town T, Mori T, Wu Y, Saxe M, Crawford F, Mullan M. CD45 opposes beta-amyloid peptide-induced microglial activation via inhibition of p44/42 mitogen-activated protein kinase. J Neurosci. 2000;20:7587–7594. doi: 10.1523/JNEUROSCI.20-20-07587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Taylor BS, Geller DA. Molecular regulation of the human inducible nitric oxide synthase (iNOS) gene. Shock. 2000;13:413–424. doi: 10.1097/00024382-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 136.Taylor BS, de Vera ME, Ganster RW, Wang Q, Shapiro RA, Morris SM, Jr, Billiar TR, Geller DA. Multiple NF-kappaB enhancer elements regulate cytokine induction of the human inducible nitric oxide synthase gene. J Biol Chem. 1998;273:15148–15156. doi: 10.1074/jbc.273.24.15148. [DOI] [PubMed] [Google Scholar]
- 137.Teng X, Zhang H, Snead C, Catravas JD. Molecular mechanisms of iNOS induction by IL-1 beta and IFN-gamma in rat aortic smooth muscle cells. Am J Physiol Cell Physiol. 2002;282:C144–152. doi: 10.1152/ajpcell.2002.282.1.C144. [DOI] [PubMed] [Google Scholar]
- 138.Trajkovic V, Stosic-Grujicic S, Samardzic T, Markovic M, Miljkovic D, Ramic Z, Mostarica Stojkovic M. Interleukin-17 stimulates inducible nitric oxide synthase activation in rodent astrocytes. J Neuroimmunol. 2001;119:183–191. doi: 10.1016/s0165-5728(01)00391-5. [DOI] [PubMed] [Google Scholar]
- 139.Vincent VA, Selwood SP, Murphy GM., Jr Proinflammatory effects of M-CSF and A beta in hippocampal organotypic cultures. Neurobiol Aging. 2002;23:349–362. doi: 10.1016/s0197-4580(01)00338-4. [DOI] [PubMed] [Google Scholar]
- 140.Walsh KA, Megyesi JF, Wilson JX, Crukley J, Laubach VE, Hammond RR. Antioxidant protection from HIV-1 gp120-induced neuroglial toxicity. J Neuroinflammation. 2004;1:8. doi: 10.1186/1742-2094-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang MJ, Huang HM, Chen HL, Kuo JS, Jeng KC. Dehydroepiandrosterone inhibits lipopolysaccharide-induced nitric oxide production in BV-2 microglia. J Neurochem. 2001;77:830–838. doi: 10.1046/j.1471-4159.2001.00295.x. [DOI] [PubMed] [Google Scholar]
- 142.Wang MJ, Jeng KC, Kuo JS, Chen HL, Huang HY, Chen WF, Lin SZ. c-Jun N-terminal kinase and, to a lesser extent, p38 mitogen-activated protein kinase regulate inducible nitric oxide synthase expression in hyaluronan fragments-stimulated BV-2 microglia. J Neuroimmunol. 2004;146:50–62. doi: 10.1016/j.jneuroim.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 143.Won JS, Im YB, Key L, Singh I, Singh AK. The involvement of glucose metabolism in the regulation of inducible nitric oxide synthase gene expression in glial cells: possible role of glucose-6-phosphate dehydrogenase and CCAAT/enhancing binding protein. J Neurosci. 2003;23:7470–7478. doi: 10.1523/JNEUROSCI.23-20-07470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Won JS, Im YB, Singh AK, Singh I. Dual role of cAMP in iNOS expression in glial cells and macrophages is mediated by differential regulation of p38-MAPK/ ATF-2 activation and iNOS stability. Free Radic Biol Med. 2004;37:1834–1844. doi: 10.1016/j.freeradbiomed.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 145.Wu J, Dent P, Jelinek T, Wolfman A, Weber MJ, Sturgill TW. Inhibition of the EGF-activated MAP kinase signaling pathway by adenosine 3′,5′-monophosphate. Science. 1993;262:1065–1069. doi: 10.1126/science.7694366. [DOI] [PubMed] [Google Scholar]
- 146.Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- 147.Xie QW, Whisnant R, Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J Exp Med. 1993;177:1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xu J, Kim GM, Chen S, Yan P, Ahmed SH, Ku G, Beckman JS, Xu XM, Hsu CY. iNOS and nitrotyrosine expression after spinal cord injury. J Neurotrauma. 2001;18:523–532. doi: 10.1089/089771501300227323. [DOI] [PubMed] [Google Scholar]
- 149.Zhang H, Chen X, Teng X, Snead C, Catravas JD. Molecular cloning and analysis of the rat inducible nitric oxide synthase gene promoter in aortic smooth muscle cells. Biochem Pharmacol. 1998;55:1873–1880. doi: 10.1016/s0006-2952(98)00078-1. [DOI] [PubMed] [Google Scholar]
- 150.Zhang J, Somani AK, Siminovitch KA. Roles of the SHP-1 tyrosine phosphatase in the negative regulation of cell signalling. Semin Immunol. 2000;12:361–378. doi: 10.1006/smim.2000.0223. [DOI] [PubMed] [Google Scholar]
- 151.Zhang Y, Kolesnick R. Signaling through the sphingomyelin pathway. Endocrinology. 1995;136:4157–4160. doi: 10.1210/endo.136.10.7664631. [DOI] [PubMed] [Google Scholar]
- 152.Zhao W, Tilton RG, Corbett JA, McDaniel ML, Misko TP, Williamson JR, Cross AH, Hickey WF. Experimental allergic encephalomyelitis in the rat is inhibited by aminoguanidine, an inhibitor of nitric oxide synthase. J Neuroimmunol. 1996;64:123–133. doi: 10.1016/0165-5728(95)00158-1. [DOI] [PubMed] [Google Scholar]


