Abstract
The activity of glutamine synthetase (EC 6.3.1.2) from Escherichia coli is regulated by the cyclic adenylylation and deadenylylation of Tyr-397 in each of the enzyme’s 12 identical subunits. The nitration of Tyr-397 or of the nearby Tyr-326 by peroxynitrite can convert the unadenylylated enzyme to a form exhibiting regulatory characteristics similar to the form obtained by adenylylation. The adenylylated conformation can also be elicited by the oxidation of surface-exposed methionine residues to methionine sulfoxide. However, the nitration of tyrosine residues and the oxidation of methionine residues are oppositely directed by the presence and absence of CO2. At physiological concentrations of CO2, pH 7.4, nitration occurs but oxidation of methionine residues is inhibited. Conversely, in the absence of CO2 methionine oxidation is stimulated and nitration of tyrosine is prevented. It was further established that adenylylation of Tyr-397 precludes its nitration by peroxynitrite. Furthermore, nitration of Tyr-326 together with either nitration or adenylylation of Tyr-397 leads to inactivation of the enzyme. These results demonstrate that CO2 can alter the course of peroxynitrite-dependent reactions and serve notice that (i) the reactions have physiological significance only if they are shown to occur at physiological concentrations of CO2 and physiological pH; and (ii) the peroxynitrite-dependent nitration of tyrosine residues or the oxidation of methionine residues of metabolically regulated proteins can seriously compromise their biological function.
The regulation of glutamine synthetase (GS; EC 6.3.1.2) activity in Escherichia coli involves the cyclic adenylylation of a particular tyrosine residue (Tyr-397) in each of the enzyme’s 12 identical subunits (1). Adenylylation of the enzyme leads to a shift in the pH–activity profile, alteration in the divalent cation specificity, and changes in the affinity of the enzyme for substrates and allosteric effectors (2). Results of earlier studies showed that nitration of tyrosine residues of the unadenylylated enzyme (GS0) by tetranitromethane (3) or by peroxynitrite (PN) (4) provokes changes in the regulatory properties similar to those elicited by adenylylation. Because the treatment with PN led not only to the nitration of tyrosine residues but also to oxidation of methionine residues to methionine sulfoxide (MetSO) residues (4), it was possible both modifications were involved in the alteration of GS regulatory properties. In the meantime it was reported that in the presence of CO2, PN is almost instantly converted to a derivative (possibly ONOOCO2−) that can mediate nitration of aromatic compounds (5). We show here that at neutral pH the PN-promoted nitration of tyrosine residues in GS is almost completely dependent on the presence of CO2, whereas the PN-mediated oxidation of methionine residues is completely prevented by CO2. We show also that the oxidation of methionine residues and the nitration of tyrosine residues can independently cause changes in the regulatory properties of GS0 that are similar to those provoked by adenylylation, and that both types of modification can lead to inactivation of the fully adenylylated GS (GS12).
MATERIALS AND METHODS
Materials.
GS was purified from E. coli YMC 10 pgln 6 as previously described (6). PN was prepared by the reaction of NaN3 with ozone as described by Pryor et al. (7). Hydrogen peroxide, 33%, was from Fisher.
Apparent State of Adenylylation.
The unadenylylated subunits of GS exhibit identical γ-glutamyltransferase activities when measured either at pH 7.57 in the presence of 0.04 mM Mn(II) or of 60 mM Mg(II), or at pH 9.0 in the presence of 0.04 mM Mn(II). Upon adenylylation, the Mn(II)-dependent activity of GS at pH 7.57 is unchanged, but the adenylylated subunits are almost completely inactive in the presence of 60 mM Mg(II) at pH 7.57, or in the presence of 0.04 Mn(II) at pH 9.0. These characteristics are the basis of highly sensitive methods (8) for measuring the state of adenylylation, n, of GS, where n is defined as the average number of adenylylated subunits in a GS dodecamer. The value of n can therefore vary from 0 to 12, depending on how many subunits are adenylylated. As shown here, the oxidation of methionine residues or the nitration of tyrosine residues in GS0 can elicit changes in GS subunits similar to those elicited by adenylylation. Because these changes in activity do not involve adenylylation, but are quantified by the same methods (8) used to measure the value of n, we refer to them as changes in the apparent state of adenylylation (napp). The value of napp is therefore a measure of conformational changes elicited by modifications of methionine or tyrosine residues that are equivalent to changes in the value of n that would be caused by adenylylation. It follows that the value of napp can also vary from 0 to 12, depending on how many subunits in the dodecamer have been converted to a form comparable to that obtained by adenylylation.
PN Treatment.
For the CO2-free experiments, 1 mg/ml GS in 50 mM phosphate buffer, pH 7.5, was purged for 5 min with N2. PN was added with a gas-tight syringe and the solution was purged another 3 min. For the experiments involving CO2, GS was incubated in 23 mM NaHCO3 and purged with 5% CO2 to obtain a pH of 7.5. Aliquots were removed for GS activity measurements and the reaction mixtures were then buffer exchanged and analyzed for nitrotyrosine (NT) and MetSO as described (4).
Identification and Quantitation of Nitrated Tyrosine Residues.
After exposure to PN, GS was carboxymethylated and cleaved by Lys-C (Sigma), and the resulting peptides were separated by reverse-phase HPLC. To carboxymethylate, the enzyme was first incubated in 6 M guanidine⋅hydrochloride/100 mM Tris/1.2 mM EDTA/5 mM dithiothreitol, pH 8.0, for 30 min at 37°C to ensure reduction of sulfhydryl groups. Then 20 mM iodoacetamide was added, and the protein was incubated another 30 min at 37°C. Dithiothreitol (15 mM) was added for 1 min at room temperature, and the GS was then dialyzed against 0.1 M Tris/1.1 mM EDTA, pH 8.5, at 4°C. Lys-C cleavage was performed on 300 μg of protein in 0.1M Tris/1.1 mM EDTA, pH 8.5 at 37°C, using 10 μg of Lys-C. After 16 hr the protein was acidified with 6 M guanidine⋅hydrochloride/0.5 M potassium phosphate, pH 2.5, and 100 μg was injected onto a Vydac C18 reverse-phase column equilibrated in 0.05% trifluoroacetic acid. The peptides were separated with an acetonitrile gradient (1%/min for 35 min, then 0.5%/min for 20 min) on a Hewlett–Packard 1090 HPLC equipped with a diode array detector. Peptides were detected by absorbance at 210, 276, and 294 nm, and nitrated peptides were detected at 360 nm. The identity of each peak was established by matrix-associated laser desorption ionization–time-of-flight (MALDI-TOF) mass spectrometry (Hewlett–Packard model G2025A) and by automated Edman sequencing (Hewlett–Packard model G1005A); both methods gave the same results. The recovery of peptides containing Trp-57 was calculated from their molar absorbtivities at 276 nm and 294 nm (which agreed) (9), and that was used to correct for any differences in recovery among HPLC runs. We found that the molar absorbtivity of NT increases linearly by ≈50% as a function of acetonitrile concentration over the range of 0–30%. Therefore, the absolute recovery of each nitrated peptide could not be calculated with confidence. Instead, the area of each peak at 360 nm was used as a measure of the extent of nitration, with the maximal area taken as complete nitration. The fractional nitration was also independently determined by the technique of simultaneous sequencing of the Lys-C digest (10), and the results agreed with those from the HPLC peptide mapping method.
RESULTS
Reciprocal Effects of CO2 on Nitration of Tyrosine and Oxidation of Methionine Residues.
When no effort is made to remove CO2 from GS reaction mixtures (phosphate buffer, pH 7.4, equilibrated with air), addition of PN leads to production of both NT and MetSO (Fig. 1). If, however, CO2 is removed from these reaction mixtures by gassing with 100% N2 prior to addition of PN, then little if any NT is formed and the yield of MetSO is increased by about 60%. Thus, the exclusion of CO2 suppresses the formation of NT and enhances the production of MetSO. The effect of gassing with N2 is not due to removal of O2 or to a unique property of N2 because identical results were obtained when the reaction mixtures were purged with 100% O2 or helium. The reciprocal effect of CO2 on nitration of tyrosine and oxidation of methionine residues is illustrated further by other data in Fig. 1 showing that high concentrations of CO2 inhibit completely the formation of MetSO and stimulate greatly the formation of NT. Even higher concentrations of phosphate or bicarbonate buffers lead to lower yields of NT (not shown).
Figure 1.
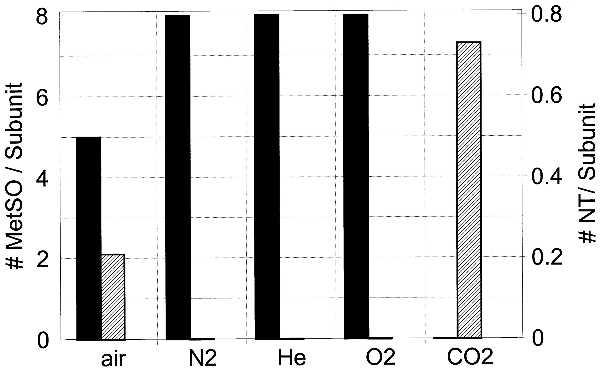
Treatment of GS with PN leads to nitration of tyrosine residues in the presence of CO2 and the oxidation of methionine residues to MetSO in the absence of CO2. Black and shaded bars represent MetSO and NT residues, respectively. One milligram of GS in 50 mM potassium phosphate, pH 7.5, was treated for 5 min with the indicated gases (100%) except in the case of CO2, for which 5% CO2, 95% N2, and 23.5 mM NaHCO3 were used to achieve pH 7.5. Reaction volume was 0.50 ml and PN was 4.0 mM. When phosphate buffer was omitted from the CO2-treated incubation, nitration increased to ≈2.0 residues per subunit.
Effect of Tyrosine Nitration and Methionine Oxidation on Apparent State of Adenylylation.
To compare the effects of nitration and methionine oxidation on the changes in the value of napp, the unadenylylated form of GS (GS0) was exposed to 0–7 mM PN in the presence or absence of CO2. The data summarized in Fig. 2 show that both the PN-dependent nitration of tyrosine residues in the presence of CO2 and the oxidation of methionine residues in the absence of CO2 are able to convert the unadenylylated form of GS to a form similar to that obtained by adenylylation. In both cases there is a linear relationship between the napp and the number of residues modified per subunit. The fact that oxidation of methionine residues alone can mimic the effects of adenylylation is confirmed by the demonstration that the oxidation of methionine residues in GS0 by H2O2 in the absence of transition metals (conditions in which only methionine residues are modified) is also able to convert the enzyme to the adenylylated conformation (Fig. 2). From extrapolations of the linear portions of the various curves in Fig. 2 it is estimated that the PN-provoked nitration of 3–4 tyrosine residues or oxidation of 6–7 methionine residues per subunit can convert GS0 to the GS12-like conformation; however, attainment of the GS12-like state by H2O2 requires the oxidation of 13–14 methionine residues per subunit. It is therefore evident that PN is either more selective than H2O2 in the oxidation of methionine residues that affect the napp changes, or that the small amount of NT (0.3–0.7 mol per subunit) formed by PN in the absence of CO2 enhances the response of napp to methionine oxidation. The latter possibility was discounted by showing that the response of napp to H2O2-mediated methionine oxidation of a GS0 preparation containing on the average 0.8 NT residue per subunit (obtained by prior treatment with 2.0 mM PN in the presence of CO2) is essentially the same as that shown in Fig. 2 for the NT-free preparation (not shown).
Figure 2.
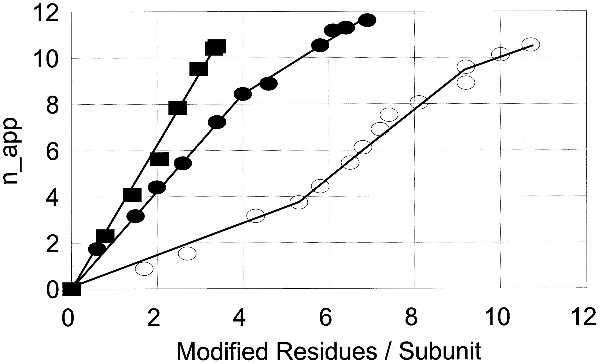
Nitration of tyrosine residues or oxidation of methionine residues converts GS to a form similar to that obtained by adenylylation. ▪, NT residues; •, MetSO formed by PN treatment; ○, MetSO formed by treatment with H2O2.
Effect of PN-mediated Nitration and Methionine Oxidation on Mn-dependent Activity of Adenylylated and Unadenylylated Forms of GS.
As noted in Materials and Methods, in the presence of Mn(II) at pH 7.57, the adenylylated and unadenylylated forms of GS exhibit equal glutamyltransferase activities. Accordingly, the transferase activity measured under these conditions is a measure of total GS activity, independent of the adenylylation status. We therefore used these assay conditions to investigate the effects of nitration and methionine oxidation by PN on the inactivation of both enzyme forms. Results summarized in Fig. 3 are from studies to determine the effect of tyrosine nitration on the activities of GS0, GS12, and a preparation of GS0 containing 11 MetSO residues per subunit (obtained by prior treatment with 160 mM H2O2). In the case of GS12, the Mn(II)-dependent activity declines linearly as a function of the number of tyrosine residues nitrated, and nitration of 3–4 tyrosine residues per subunit leads to complete loss of transferase activity. An identical pattern of inactivation was observed for the preparation of GS0 containing 11 MetSO residues. However, in the case of unmodified GS0, nitration of up to 1.5 tyrosine residues per subunit had no effect on the Mn(II)-dependent activity; but nitration of additional tyrosine residues led to loss of activity in parallel to that observed for nitration of the adenylylated enzyme. It should be noted that although nitration of more than 1.5 tyrosine residues per subunits leads to a loss of GS activity, the value of napp of the residual enzyme activity continues to increase linearly with increasing nitration over the range of 1.5 to 4.0 residues per subunit (Fig. 2). In summary, the data in Fig. 3 indicate that the unadenylylated form of GS can be converted to a form exhibiting GS12-like sensitivity either by oxidation of 11 of the 16 methionine residues or by nitration of 1.5–2.0 of the 17 tyrosine residues per subunit.
Figure 3.
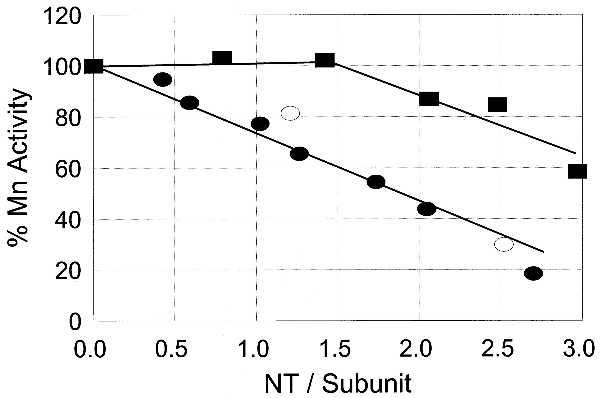
Effect of nitration on the Mn(II)-dependent transferase activity of adenylylated, unadenylylated, and oxidized unadenylylated GS. ▪, unadenylylated GS; •, adenylylated GS; ○, unadenylylated GS containing 11 MetSO residues obtained by reaction with H2O2.
Effect of H2O2-Mediated Oxidation of Methionine Residues on the Activity of Adenylylated and Unadenylylated Forms of GS.
As illustrated in Fig. 4, the oxidation of up to 10–11 methionine residues of unadenylylated GS by H2O2 has little or no effect on the Mn(II)-dependent activity—i.e., on inactivation of the enzyme. In contrast, the activity of the adenylylated enzyme (GS12) declines linearly as a function of the number of methionine residues oxidized, over the range of 0 to 8.0 residues per subunit. Moreover, activity of a GS0 preparation containing only one NT per subunit (obtained by prior incubation with 2.0 mM PN) is more sensitive to methionine oxidation than is the unmodified GS0.
Figure 4.
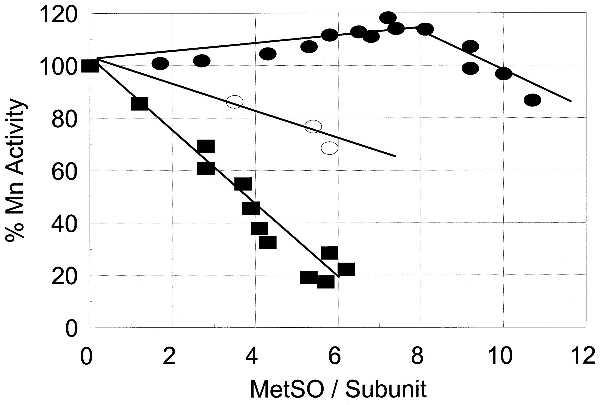
Effect of methionine oxidation by H2O2 on the Mn(II)-dependent activity of adenylylated and unadenylylated GS containing one NT residue per subunit. •, unadenylylated GS; ▪, adenylylated GS; ○, unadenylylated GS containing 1.0 NT residue per subunit.
Comparison of the Ability of PN to Nitrate Unadenylylated and Adenylylated Forms of GS.
Data summarized in Fig. 5 show that the adenylylated and unadenylylated forms of GS are almost equally susceptible to nitration by various concentrations of PN. This finding is surprising in view of the fact that adenylylated tyrosine residues should be resistant to nitration, and it raises the question of whether the effects of nitration on the napp of GS0 are due to the nitration of tyrosine residues other than the tyrosine that is involved in the adenylylation reaction. To study this further we exposed preparations of GS0 and a preparation of GS containing 10 adenylyl groups (i.e., GS10) to PN under conditions (pH 7.4, 5% CO2 atmosphere) where about 2.0 tyrosine residues per subunit were nitrated. The preparations were then treated with snake venom phosphodiesterase (SVD) to remove the adenylyl groups from the enzyme (11), and the effects of these treatments on the napp were measured. The results (Table 1) show the following: (i) Nitration of about 2 tyrosine residues per subunit converts GS0 to a form that is similar to that obtained by adenylylation of 4 tyrosine residues per dodecamer, but has no effect on the n value of GS10. (ii) Treatment of either the unmodified or nitrated forms of GS0 with SVD has no effect on the napp value. (iii) Treatment of the unnitrated form of GS10 with SVD converts the enzyme to the GS0 conformation, as expected, whereas treatment of the nitrated GS10 with SVD converts it to a form that is indistinguishable from that obtained by the nitration of GS0. These results and those described below demonstrate that under conditions where only 2 tyrosine residues per subunit are nitrated, the tyrosine residues that are nitrated in adenylylated GS are different from those that are nitrated in the unadenylylated form of GS. In the case of GS0, one of the two nitrated tyrosines is Tyr-397, which is the tyrosine that undergoes the enzyme-catalyzed reaction; in the case of adenylylated GS, Tyr-397 is not nitrated. Thus, although different pairs of tyrosine residues are nitrated, the effect of nitration on the napp of GS10 (after removal of the adenylyl groups by SVD) is the same as that obtained by nitration of GS0 (Table 1).
Figure 5.
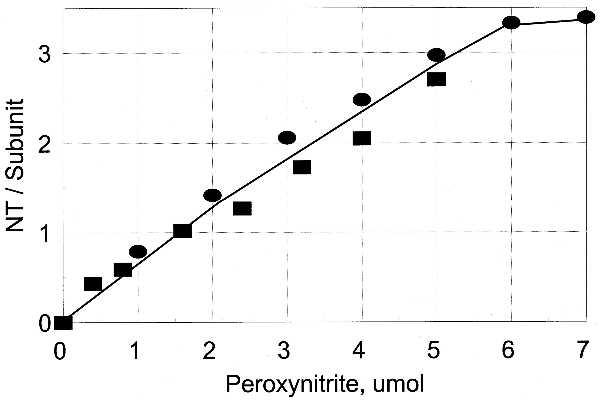
Unadenylylated and adenylylated GS are almost equally susceptible to tyrosine nitration by PN. •, GS0; ▪, GS12. PN was varied from 0 to 7 mM. The reaction volume was 1.0 ml. The solution was gassed for 5 min, PN was added, and gassing was continued for another 3 min.
Table 1.
Effects of nitration and SVD on napp of adenylylated and unadenylylated forms of GS
| GS preparation | NT/subunit | napp |
|---|---|---|
| GS0 | 0 | 0.1 |
| GS0 + SVD | 0 | 0.3 |
| GS0 + PN | 2.0 | 4.3 |
| GS0 + PN + SVD | 2.0 | 4.1 |
| GS10 | 0 | 10.0 |
| GS10 + SVD | 0 | 0.2 |
| GS10 + PN | 1.7 | 10.3 |
| GS10 + PN + SVD | 1.7 | 4.3 |
Samples (1 mg) of GS preparations containing zero (GS0) and 10 (GS10) adenylyl groups per dodecamer were treated with 2.5 mM PN in the presence of CO2, leading to the nitration of 2.0 and 1.7 NT residues per subunit, respectively. These and untreated controls were then treated with 0.8 μg of SVD to remove the adenylyl groups (11), and the values of napp were determined by measurement of the transferase activities in the presence of Mn or Mg.
Identification of Nitrated Tyrosines in Unadenylylated and Adenylylated GS.
The nitration of each of the 17 tyrosine residues was assessed by reverse-phase peptide mapping and by simultaneous sequencing of peptides, with both methods giving the same results. Tyr-397 is the adenylylated residue in GS, and it was very susceptible to nitration when unadenylylated (GS0) but essentially resistant when adenylylated (GS12). Except for this expected difference, the susceptibility to nitration of each of the other tyrosine residues was similar in both unadenylylated and adenylylated forms of GS (Figs. 6 and 7, and Table 2). Tyrosines 287, 296, and 326 were readily nitrated; tyrosines 100 and 114 were less susceptible; tyrosine 179 was only slightly susceptible in the unadenylylated GS0; all other tyrosines were effectively resistant to nitration. The susceptibility of methionine residues in GS to oxidation by hydrogen peroxide correlates very well with their surface exposure (10). Examination of the structure derived by Eisenberg and colleagues (12) shows that susceptibility to nitration also requires surface exposure of the carbon atoms (Table 2, Fig. 8). However, surface exposure alone does not account completely for the relative susceptibility, in particular the somewhat lesser susceptibility of Tyr-100 and Tyr-114 and the low susceptibility of Tyr-334.
Figure 6.
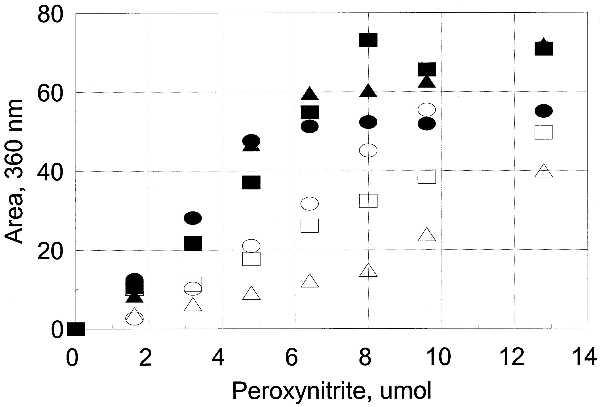
Susceptibility of individual tyrosine residues of GS0 to nitration. The area of the nitrated peaks for those tyrosines that were susceptible to nitration (Table 2) was obtained from the HPLC peptide map as described in the text. A single peptide contained both Tyr-287 and Tyr-296, which were both shown by simultaneous sequencing to be rapidly nitrated, so the area for that peptide was divided by 2 before plotting. ▪, Tyr-397; •, Tyr-326; ▴, Tyr-287/Tyr-296; □, Tyr-114; ○, Tyr-100; ▵, Tyr-179.
Figure 7.
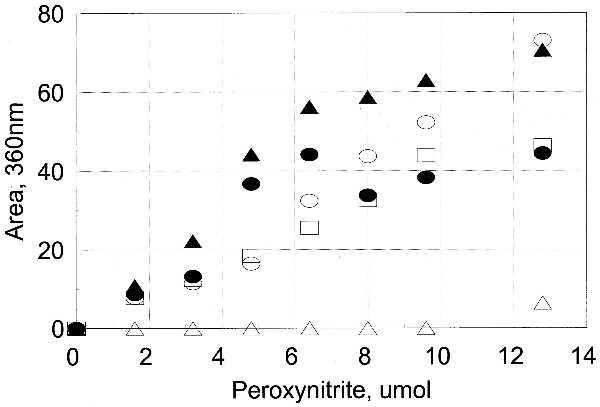
Susceptibility of individual tyrosine residues of GS12 to nitration. The area of the nitrated peaks for those tyrosines that were susceptible to nitration (Table 2) was obtained from the HPLC peptide map as described in the text. A single peptide contained both Tyr-287 and Tyr-296, which were both shown by simultaneous sequencing to be rapidly nitrated, so the area for that peptide was divided by 2 before plotting. •, Tyr-326; ▴, Tyr-287/Tyr-296; □, Tyr-114; ○, Tyr-100; ▵, Tyr-179.
Table 2.
Nitration of tyrosine residues in GS
| Tyr | Susceptibility to nitration
|
Relative susceptibility: GS0 vs. GS12 | Surface exposure
|
||
|---|---|---|---|---|---|
| GS0 | GS12 | CE1* | CE2* | ||
| 100 | Moderate | Moderate | Equal | Yes | No |
| 114 | Moderate | Moderate | Equal | Slight | Yes |
| 164 | ≈Nil | ≈Nil | Equal | Slight† | Yes† |
| 179 | Low | Slight | GS0 > GS12 | No | No |
| 238 | ≈Nil | ≈Nil | Equal | No | No |
| 240 | ≈Nil | ≈Nil | Equal | Yes† | No |
| 287 | High | High | Equal | Yes | Yes |
| 296 | High | High | Equal | Yes | Yes |
| 297 | ≈Nil | ≈Nil | Equal | No | No |
| 319 | ≈Nil | ≈Nil | Equal | No | No |
| 326 | High | High | Equal | Yes | No |
| 334 | Low | Low | Equal | No | Yes |
| 368 | ≈Nil | ≈Nil | Equal | No | No |
| 397 | High | ≈Nil | GS0 ≫ GS12 | Yes | Yes |
| 442 | ≈Nil | ≈Nil | Equal | No | No |
| 465 | ≈Nil | ≈Nil | Equal | No | No |
| 466 | ≈Nil | ≈Nil | Equal | No | No |
CE1 and CE2 are the two carbons at the 3 position of the tyrosyl ring, either of which is potentially nitratable by peroxynitrate.
This atom faces the central cavity, an orientation that may inhibit attack by PN (see Fig. 8).
Figure 8.
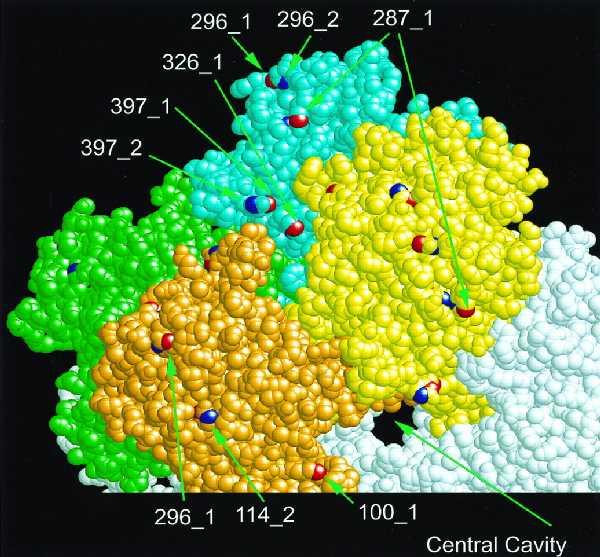
Location of surface-exposed tyrosines in GS. This figure was created by the program RasMol (18), using the coordinates determined by Almassy et al. (12) and deposited in the Brookhaven Data base as 2GLS. Each molecule consists of 12 identical subunits arranged as two hexamers. In this view, two adjacent subunits from the upper hexamer are colored green and blue while the two from the lower hexamer are orange and yellow; other subunits are white. Looking at the orange and blue subunits allows visualization of the “front” and “back” of the subunit. Tyrosine is nitrated at the 3 position of the ring, and the two susceptible carbons are named CE1 and CE2 in the crystallographic nomenclature. In each colored subunit, the 17 CE1 atoms are colored red and the 17 CE2 atoms are blue; only those that are surface exposed are visible in this space-filling model. The exposed tyrosines are identified by their residue number and a 1 or 2 for CE1 or CE2.
The inactivation of adenylylated GS12 by PN correlates with the nitration of the highly susceptible residues, indicating that nitration of Tyr-287, Tyr-296, or Tyr-326 causes the inactivation (Fig. 9). Tyr-326 is very close to the adenylylated Tyr-397, suggesting that the interaction of the two modified residues leads to loss of catalytic activity. This possibility is supported by the recovery of activity that occurred when the adenylyl group was removed by phosphodiesterase treatment (Table 1). If modification of both residues is required, either by adenylylation or by nitration, then the pattern of inactivation of the unadenylylated enzyme should be sigmoidal. This is the pattern that was observed, and the fraction inactivated matched the fraction of protein that was nitrated on both Tyr-326 and Tyr-397 (Fig. 10). Inactivation is thus a consequence of the covalent modification of two tyrosyl residues that are close to each other in the folded protein. This finding also demonstrates that adenylylation mimics nitration, just as nitration was shown previously to mimic adenylylation.
Figure 9.
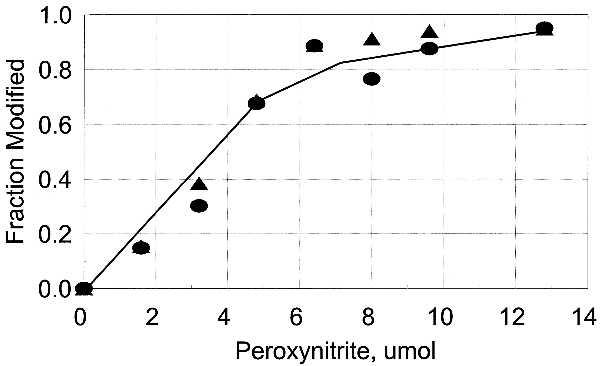
Inactivation of adenylylated GS correlates with nitration of Tyr-326. ▴, fraction of activity that is lost; •, fraction of Tyr-326 that is nitrated.
Figure 10.
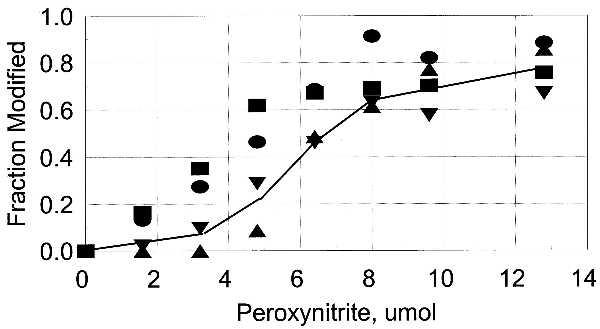
Inactivation of unadenylylated GS correlates with nitration of both Tyr-326 and Tyr-397. ▪, fraction of Tyr-326 that is nitrated; •, fraction of Tyr-397 that is nitrated; ▾, fraction in which both Tyr-397 and Tyr-326 are nitrated; ▴, fraction of GS0 that is inactive.
DISCUSSION
It is evident from the data presented here that the nitration of tyrosine residues or oxidation of methionine residues by PN can convert a GS subunit to a form that is similar to that obtained by adenylylation of a single tyrosine residue (Tyr-397) in the subunit. The ability of PN to nitrate tyrosine residues and to oxidize methionine residues is strictly dependent on the availability of CO2. In the absence of CO2, PN oxidizes surface-exposed methionine residues but has little or no ability to nitrate tyrosine residues. Conversely, in the presence of physiological concentrations of CO2 (pH 7.4), nitration occurs but methionine oxidation is inhibited. In accordance with results of Pryor et al. (13) showing that the oxidation of Met to MetSO involves conversion of PN to an activated form (PN*) of undetermined structure, and results of studies (5, 14–16), showing that PN reacts with CO2 to form an unstable derivative (possibly ONOOCO−) that can nitrate tyrosine residues, we propose that the oxidation of methionine residues and the nitration of tyrosine residues of GS are reciprocally controlled by the availability of CO2, as illustrated in Fig. 11. In recent years there have been numerous reports describing the interaction of PN with biological molecules. The demonstration here that CO2 can alter the course and specificity of PN-mediated reactions serves notice that the physiological significance of these reactions in mammals is suspect, unless they occur in the presence of physiological concentrations of CO2, that is, in the presence of 5% CO2 at pH 7.4.
Figure 11.
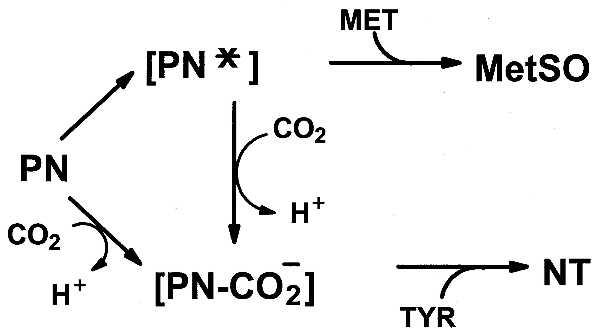
Effect of CO2 on nitration and methionine oxidation by PN. CO2 favors nitration of tyrosine and suppresses oxidation of methionine.
On the basis of the known crystal structure of GS (12) and the present studies using MALDI-TOF mass spectrometry and Edman sequencing of proteolytic fragments of PN-modified GS0 and GS12 preparations, it was possible to identify those tyrosine residues that are most susceptible to nitration. From such studies it was established that the nitration of a tyrosine residue converts an unadenylylated subunit to the adenylylated-like conformation, and that adenylylation of Tyr-397 precludes its nitration by PN. Furthermore, it is clear that nitration of Tyr-326 together with either nitration or adenylylation of Tyr-397 leads to complete inactivation of GS.
Previous studies showed that nitration of a tyrosine residue in a model peptide substrate prevented phosphorylation of this tyrosyl residue by the lck protein kinase (17). Taking this finding together with the results described here, it is clear that the regulatory properties of enzymes or proteins whose activities are regulated by esterification of tyrosine residues, as occurs in signal transduction networks, can be seriously compromised by PN-mediated nitration or methionine oxidation.
ABBREVIATIONS
- GS
glutamine synthetase
- GS0
unadenylylated glutamine synthetase
- GS10–12
adenylylated glutamine synthetase
- n
number of adenylylated subunits
- napp
the apparent number of adenylylated subunits caused by PN modifications that convert glutamine synthetase to a conformation analogous to that obtained by adenylylation of n subunits
- NT
nitrotyrosine
- MetSO
methionine sulfoxide
- PN
peroxynitrite
- MALDI-TOF
matrix-associated laser desorption ionization–time-of-flight
- SVD
snake venom phosphodiesterase
References
- 1.Stadtman E R, Chock P B, Rhee S G. Curr Top Cell Regul. 1981;18:79–94. doi: 10.1016/b978-0-12-152818-8.50011-6. [DOI] [PubMed] [Google Scholar]
- 2.Stadtman E R, Ginsburg A. In: The Enzymes. Boyer P D, editor. New York: Academic; 1974. pp. 755–807. [Google Scholar]
- 3.Cimino F, Anderson W B, Stadtman E R. Proc Natl Acad Sci USA. 1970;66:564–571. doi: 10.1073/pnas.66.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berlett B S, Friguet B, Yim M B, Chock P B, Stadtman E R. Proc Natl Acad Sci USA. 1996;93:1776–1780. doi: 10.1073/pnas.93.5.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lymar S V, Hurst J K. J Am Chem Soc. 1995;117:8867–8868. [Google Scholar]
- 6.Miller R E, Shelton E, Stadtman E R. Arch Biochem Biophys. 1974;163:155–171. doi: 10.1016/0003-9861(74)90465-2. [DOI] [PubMed] [Google Scholar]
- 7.Pryor W A, Cueto R, Jin X, Koppenol WH, Ngu-Schwemlein M, Squadrito G L, Uppu P L, Uppu R M. Free Radical Biol Med. 1995;18:75–83. doi: 10.1016/0891-5849(94)00105-s. [DOI] [PubMed] [Google Scholar]
- 8.Stadtman E R, Smyrniotis P Z, Davis J N, Wittenberger M E. Anal Biochem. 1979;95:275–285. doi: 10.1016/0003-2697(79)90217-3. [DOI] [PubMed] [Google Scholar]
- 9.Levine R L, Williams J A, Stadtman E R, Shacter E. Methods Enzymol. 1994;233:346–357. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- 10.Levine R L, Mosoni L, Berlett B S, Stadtman E R. Proc Natl Acad Sci USA. 1996;93:15036–15040. doi: 10.1073/pnas.93.26.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shapiro B M, Kingdon H S, Stadtman E R. Proc Natl Acad Sci USA. 1967;58:642–649. doi: 10.1073/pnas.58.2.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almassy R J, Janson C A, Hamlin R, Xuong N H, Eisenberg D. Nature (London) 1986;323:304–309. doi: 10.1038/323304a0. [DOI] [PubMed] [Google Scholar]
- 13.Pryor W A, Jin X, Squadrito G L. Proc Natl Acad Sci USA. 1994;91:11173–11177. doi: 10.1073/pnas.91.23.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lymar SV, Jiang Q, Hurst J K. Biochemistry. 1996;35:7855–7861. doi: 10.1021/bi960331h. [DOI] [PubMed] [Google Scholar]
- 15.Uppu R M, Squadrito G L, Pryor W A. Arch Biochem Biophys. 1996;327:335–343. doi: 10.1006/abbi.1996.0131. [DOI] [PubMed] [Google Scholar]
- 16.Denicola A, Freeman B A, Trujillo M, Radi R. Arch Biochem Biophys. 1996;333:49–58. doi: 10.1006/abbi.1996.0363. [DOI] [PubMed] [Google Scholar]
- 17.Kong S K, Yim M B, Stadtman E R, Chock P B. Proc Natl Acad Sci USA. 1996;93:3377–3382. doi: 10.1073/pnas.93.8.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sayle R A, Milner-White E J. Trends Biochem Sci. 1995;20:374–374. doi: 10.1016/s0968-0004(00)89080-5. [DOI] [PubMed] [Google Scholar]


