Abstract
The yeast Golgi membrane protein Rer1p is required for the retrieval of various endoplasmic reticulum (ER) membrane proteins such as Sec12p and Sec71p to the ER. We demonstrate here that the transmembrane domain (TMD) of Sec71p, a type-III membrane protein, contains an ER localization signal, which is required for physical recognition by Rer1p. The Sec71TMD-GFP fusion protein is efficiently retrieved to the ER by Rer1p. The structural feature of this TMD signal turns out to be the spatial location of polar residues flanking the highly hydrophobic core sequence but not the whole length of the TMD. On the Rer1p side, Tyr152 residue in the 4th TMD is important for the recognition of Sec12p but not Sec71p, suggesting that Rer1p interacts with its ligands at least in two modes. Sec71TMD-GFP expressed in the Δrer1 mutant cells is mislocalized from the ER to the lumen of vacuoles via the multivesicular body (MVB) sorting pathway. In this case, not only the presence of polar residues in the Sec71TMD but also the length of the TMD is critical for the MVB sorting. Thus, the Rer1p-dependent ER retrieval and the MVB sorting in late endosomes both watch polar residues in the TMD but in a different manner.
INTRODUCTION
The secretory pathway of eukaryotic cells consists of a series of discrete membrane-bounded organelles with distinct protein and lipid compositions. Each protein that functions in a particular organelle must have a specific signal for its localization. Transmembrane domains (TMDs) of membrane proteins have often been shown to contain important information for localization in the ER (Bonifacino et al., 1990a, 1990b, 1991; Pedrazzini et al., 1996, 2000; Sato et al., 1996; Rayner and Pelham, 1997; Honsho et al., 1998; Letourneur and Cosson, 1998; Massaad et al., 1999) and the Golgi complex (Munro, 1995), for transport between the Golgi and endosomes (Lewis et al., 2000) or the plasma membrane (Scheiffele et al., 1997), for sorting between endosomes (Reggiori et al., 2000; Zaliauskiene et al., 2000), and for endocytosis (Zaliauskiene et al., 2000). Despite such a variety of studies, molecular mechanisms of how these TMDs are recognized during sorting are still largely unknown.
A clue to understand such membrane protein sorting was obtained from our work on the Rer1p-dependent localization of ER membrane proteins. Rer1p is a Golgi-resident membrane protein of 188 amino acid residues containing four membrane-spanning domains and is required for the ER localization of the type-II membrane protein Sec12p (Nishikawa and Nakano, 1993; Boehm et al., 1994; Sato et al., 1995). The TMD of Sec12p contains an Rer1p-dependent retrieval signal from the Golgi to the ER (Sato et al., 1996). Recently, we demonstrated that Rer1p physically recognizes the TMD of Sec12p and returns Sec12p to the ER via the COPI vesicles (Sato et al., 1996, 1997, 2001). Besides Sec12p, Rer1p is required for localization of a variety of ER membrane proteins such as Sed4p, Mns1p, Sec71p, and Sec63p (Sato et al., 1996, 1997; Massaad et al., 1999). Sed4p (Hardwick et al., 1992) and Mns1p (Camirand et al., 1991) are type-II membrane proteins. Sec71p is type III (Feldheim et al., 1993; Kurihara and Silver, 1993), and Sec63p spans the ER membrane three times (Rothblatt et al., 1989; Sadler et al., 1989). Sec71p and Sec63p form a multimeric complex required for the posttranslational translocation of newly synthesized secretory proteins (Deshaies et al., 1991). Some mutant versions of Gas1p and Ste2p also show Rer1p-dependent ER localization (Letourneur and Cosson, 1998).
Another interesting example of membrane protein sorting is seen during the formation of lumenal small vesicles in late endosomes, which is known as multivesicular body (MVB) sorting (Odorizzi et al., 1998). A key molecule in this sorting is the Golgi-membrane–localized ubiquitin ligase Tul1p, which ubiquitinates a subset of membrane proteins like Cps1p and Phm5p and by doing so makes them sorted into MVBs (Reggiori and Pelham, 2002). Polar residues in the TMD of these membrane proteins have been shown important to be ubiquitinated by Tul1p (Reggiori et al., 2000, Reggiori and Pelham, 2002).
In the present study, we performed intensive analyses of the structural features of membrane protein recognition during the Rer1p-dependent ER retrieval using Sec71p as the representative ligand. We present evidence that the TMD of Sec71p not only contains a signal to bind to Rer1p but is also recognized by the MVB sorting mechanism in a different manner when it escapes from Rer1p.
MATERIALS AND METHODS
Yeast Strains and Culture Condition
Saccharomyces cerevisiae strains used are listed in Table 1. Cells were grown in the MVD medium [0.67% yeast nitrogen base without amino acids (Difco Laboratories Inc.), and 2% glucose] or MCD, which is MVD containing 0.5% casamino acids (Difco Laboratories Inc.), supplemented appropriately.
Table 1.
Yeast strains used in this study
| Strain | Genotype |
|---|---|
| SNY9a | MATα mfα1 :: ADE2 mfα2 :: TRP1 bar1 :: HIS3 ura3 leu2 trp1 his3 lys2 ade2 |
| SKY5b | MATα rer1-2 mfα1 :: ADE2 mfα2 :: LEU2 bar1 :: HIS3 ura3 leu2 trp1 his3 lys2 ade2 |
| SKY7b | MATα rer1 :: LEU2 mfα1 :: ADE2 mfα2 :: TRP1 bar1 :: HIS3 ura3 leu2 trp1 his3 lys2 ade2 |
| SKY27c | MATα ret1-1 mfα1 :: ADE2 mfα2 :: TRP1 bar1 :: HIS3 ura3 leu2 trp1 his3 lys2 ade2 |
| SMY22-10Bd | MATadap2 :: LEU2 mfα1 :: ADE2 bar1 :: HIS3 ura3 leu2 trp1 his3 his4 ade2 |
| BC180e | MATasst2-2 ura3 leu2 his3 ade2 |
| SKY75f | MATα rer1 :: LEU2 mfα1 :: ADE2 mfα2 :: HIS3 bar1 :: HIS3 ura3-52 trp1-ξ901 his3-ξ200 lys2-801 suc2-ξ9 |
| SKY80f | MATα ξvps27 :: HIS3 ξrer1 :: LEU2 ura3-52 lys2-801amber ade2-101ochre trp1-ξ63 his3-ξ200 leu2-ξ1 |
| SKY42-12Df | MATα rer1 :: LEU2 pep4 :: ADE2 ura3 leu2 trp1 his |
Source or reference: a Nishikawa and Nakano, 1993; b Sato et al., 1995; c Sato et al., 1997; d Sato et al., 2001; e W. Courchesne; and f this study.
Plasmid Construction
Construction of SEC12-MFα1 (S12M), MFα1-SEC71-HA (MS71H), and MFα1-SEC63-myc (MS63F) was described previously (Nishikawa and Nakano, 1993; Sato et al., 1997). Chimeric genes, which consist of MFα1, WBP1, and SEC71, were constructed as follows. The 800-base DNA fragment, which contains the coding sequence of the C-terminal region of Wbp1p (154 amino acid residues) followed by the WBP1 terminator, was synthesized by genomic PCR with WBP1-1 (5′-GGGTTCGAAACTAGTTATGACGAAGAGCCC-3′) and WBP1–2 (5′-GGGCTCGAGATGTTACAGGATGATAGGTGG-3′). This fragment was digested with BstBI and XhoI and used to replace the BstBI-XhoI region of MS63L containing the SEC63 ORF on pBluescript II KS+ (Sato et al., 1997), to produce pBluescript II KS+/SEC63 promoter-MFα1–WBP1-WBP1 terminator. Then, the NheI-digested DNA fragment encoding three copies of the HA epitope (YPYDVPDYA) was inserted into the SpeI site just after the BstBI site between MFα1 and WBP1 to give pMWWWK. Mutations, which change the dilysine signal KKTN at the C termini of the MWWWK protein to SSTN, were introduced by PCR-mediated mutagenesis to make pMWWWS. To construct various chimeras of MWWWS with SEC71, BglII and HindIII sites were introduced adjacent to the TMD by PCR. Introduction of these sites did not change the amino acid sequence of MWWWS. DNA fragments encoding the lumenal, transmembrane, and cytoplasmic domains of Sec71p were synthesized by PCR and used to replace the corresponding regions of MWWWS, resulting in M71WWS, MW71WS, MWW71, and M71W71. These chimeric genes were subcloned into the single-copy plasmid pRS316 (Sikorski and Hieter, 1989). The plasmids for the mutational analysis of the Sec71pTMD were also constructed by PCR and subcloned into pRS316.
To insert the GFP peptide at the C-terminus of Sec71p, the SEC71-B construct was utilized, which contained BstBI and NheI sites at the ends of the SEC71 ORF (Sato et al., 1997). The ORF of GFP in pEGFP-1 (Clontech Laboratories, Inc.) was synthesized by PCR with primers, EGFP-SpeI+ (5′-GACTAGTATGGTGAGCAAGGGCG-3′) and EGFP-SpeI-(5′-GACTAGTCTTGTACAGCTCGTCC-3′). The GFP fragment was digested with SpeI and inserted into the NheI site of pBluescript IIKS+/SEC71-B. The resulting SEC71-GFP fusion was subcloned into pRS316 and pSQ326. To construct the SEC71TMD-GFP fusion gene, the GFP ORF in pEGFP-1 was amplified by PCR with primers, GFP-NheI+ [5′-CTAGCTAGCATGGTGAGCAAGGGCGAGGAGTTACTTGTACAGCTCCTC-3′] and EGFP-SpeI–. The NheI/SpeI-digested GFP fragment was inserted into the NheI site of pBluescript IIKS+/SEC71-B to make pKS/SEC71-GFP-NheI. DNA fragments encoding the Sec71p TMD or its mutants were synthesized by PCR using MW71WS or its mutants as a template and primers, SEC71TMD-ClaI+ [5′-CCATCGATGAGACGAAATCAATCTCCGTTTATACCCCA-3′] and SEC71TMD-NheI-[5′-CTAGCTAGCTTTTTTGGCCTGCTTCTTTCTGTAGCTTGA-3′]. The ClaI/NheI-digested SEC71TMD fragments were inserted into the BstBI/NheI-digested pKS/SEC71-GFP-NheI to replace the SEC71 ORF by SEC71TMD coding sequence. The SEC71TMD-GFP gene was subcloned into pRS306 and pRS316 (Sikorski and Hieter, 1989). pRS306 harboring SEC71TMD-GFP or its derivatives were cut with StuI and introduced into wild-type (SNY9) and Δrer1 (SKY7) cells.
Construction of GFP-RER1 was described previously (Sato et al., 2001). Mutant forms of the RER1 gene were constructed by PCR and subcloned into the BglII site of pSKY5 (Sato et al., 2001). RER1-3HA Y152L mutant was also made by PCR-mediated mutagenesis using RER1-3HA as a template (Sato et al., 1995).
Antibodies
Anti-Dap2p and anti-GFP polyclonal antibodies were provided by Y. Amaya of Niigata University and H. Abe of our laboratory, respectively. Monoclonal antibodies against the HA epitope, 12CA5 and 16B12, were purchased from Boehringer and Berkeley Antibody Company, respectively. Anti-HA polyclonal antibody (Y11) and anti-GFP mAb were obtained from Santa-Cruz Biotechnology, Inc. (Santa Cruz, CA), Medical and Biological Laboratories Co. Ltd., and Clontech Laboratories, Inc., respectively.
Confocal Laser Microscopy
GFP fluorescence was visualized under an Olympus BX-60 fluorescence microscope equipped with a confocal laser scanner unit CSU10 (Yokogawa Electronic Corp.). Images were acquired by a high-resolution digital CCD camera (Hamamatsu Photonics, C4742–95), and processed by the IPLab software (Scanalytics Inc.).
Halo Assay
Halo assay was performed on MCD plates with a tester MATa sst2 strain (BC180) as described previously (Nishikawa and Nakano, 1993; Hopkins et al., 2000). The cells of BC180/pRS316 (∼5 × 105/plate) were spread on MCD plates buffered at pH 3.5. For quantification of the secreted α factor, various amounts (1, 5, 10, 50 ng) of synthetic α-factor (Peptide Institute Inc.) were spotted on filter-paper disks placed on the sst2 tester lawn. Measuring the radii of the halos and plotting them against the amount of α-factor established a standard profile of α-factor secretion (Hopkins et al., 2000). Cells to be tested for α-factor production were spotted onto the BC180 plates and then incubated at 23°C for 48 h. According to the standard profile, eight or four independent transformants for each construct were examined to quantify the amount of α-factor secreted.
Cross-link Experiments
Cross-linking between Rer1p and its ligand was performed using the thiol-cleavable linker dithiobis (succinimidyl propionate; DSP) as described previously (Sato et al., 2001). In a typical experiment, cells coexpressing Rer1-3HAp or Rer1Y152L-3HAp and Sec71-GFP or Sec71TMD-GFP were spheroplasted, lysed in 25 mM Na phosphate (pH 7.2), and incubated with 5 mM DSP at 20°C for 30 min. Reactions were terminated by the addition of 50 mM Tris HCl (pH 8.0), and then membranes were solubilized with 1% Triton X-100. After adjustment to 35 mM Tris-HCl (pH 8.0)/120 mM NaCl/2% SDS, the samples were heated to 75°C for 10 min and processed for immunoprecipitation with the anti-GFP polyclonal antibody and the anti-HA mAb (12CA5). The immunoprecipitates were treated with 5% β-mercaptoethanol to cleave DSP and then analyzed by immunoblotting with the anti-GFP monoclonal or polyclonal antibody, and the anti-HA monoclonal (16B12) or polyclonal (Y11) antibody. Interaction of Rer1p or Rer1Y152L-3HAp with DSD was also assessed as described previously (Sato et al., 2001).
RESULTS
GFP-Sec12p and Sec71p-GFP Are Localized to the ER by the Rer1p-dependent Retrieval
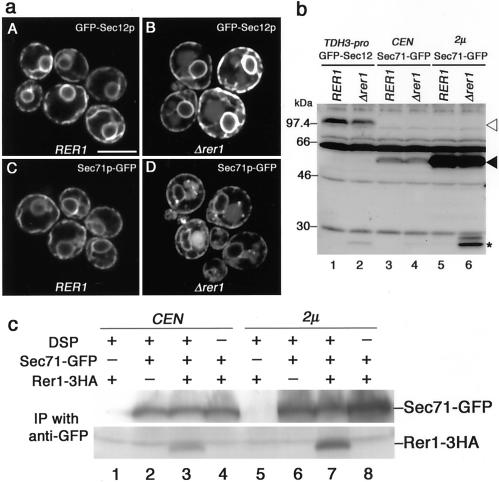
The Rer1p-dependent ER localization of Sec12p and Sec71p that we showed previously (Sato et al., 1997) was confirmed by a more straightforward microscopic approach. GFP was fused to the NH2-terminus of Sec12p and to the COOH-terminus of Sec71p. GFP-Sec12p and Sec71p-GFP complemented Δsec12 and Δsec71 mutants, respectively, indicating that they are fully functional (our unpublished results). These fusions were expressed in the wild-type and Δrer1 cells and examined by confocal laser scanning microscopy (Figure 1a). As expected, GFP-Sec12p and Sec71p-GFP were almost exclusively localized to the ER in the wild-type cells. The fluorescence shown in panels A and C visualizes the nuclear envelope and peripheral ER, typical for the yeast ER. In the Δrer1 cells, in contrast, some portion of GFP-Sec12p and Sec71p-GFP were mislocalized to the vacuole (Figure 1a, B and D). The presence of fluorescence signals in the vacuolar lumen rather than the vacuolar membrane is noteworthy because the GFP moiety of these fusions is expected to face the cytoplasm. It is reminiscent of carboxypeptidase S (CPS), which is transported to the vacuole via the multivesicular body (MVB) sorting pathway (Odorizzi et al., 1998). The GFP-CPS fusion protein is internalized into the lumen of late endosomes via small vesicles and eventually ends up in the vacuolar lumen. Indeed in the immunoblotting analysis (Figure 1b), GFP-Sec12p (open arrowhead) and Sec71-GFP (closed arrowhead) remained intact in the wild-type cells, but part of them were processed to a 27-kDa species (asterisk, perhaps cleaved GFP) in the Δrer1 cells. This degradation is due to vacuolar proteases, because it was not observed in Δrer1 pep4 mutant cells. This problem is pursued later.
Figure 1.
Rer1p-dependent ER localization of GFP-Sec12p and Sec71p-GFP. (a) Subcellular localization of GFP-Sec12p and Sec71p-GFP. The wild-type (SNY9) and Δrer1 (SKY7) cells were transformed with a single-copy plasmid containing GFP-SEC12 (TDH3 promoter) or SEC71-GFP (SEC71 promoter) and subjected to confocal laser scanning microscopy. Bright ring-shaped structures are nuclear envelopes, a part of the ER, and fuzzy large organelles seen in the Δrer1 mutant are vacuoles. (b) Immunoblotting analysis of GFP-Sec12p and Sec71p-GFP. Wild-type (SNY9) and Δrer1 (SKY7) cells expressing GFP-Sec12p under the TDH3 promoter or Sec71p-GFP under the own promoter on a single-copy (CEN) or multicopy (2 μ) plasmid were grown at 20°C. Cell extracts were subjected to immunoblotting with the anti-GFP antibody. Open arrowhead, GFP-Sec12p; closed arrowhead, Sec71p-GFP; asterisk, degradation product. (c) Physical interaction between Rer1p and Sec71p. Cell lysates of the Δrer1 Δpep4 strain (SKY42) expressing Rer1-3HAp on a multicopy plasmid and Sec71p-GFP on a single-copy (CEN) or multicopy (2 μ) plasmid were subjected to chemical cross-linking with DSP. The immunoprecipitates with the anti-GFP antibody were examined by immunoblotting with anti-GFP and anti-HA antibodies.
Next, we performed chemical cross-linking experiments using a thiol-cleavable linker DSP to prove physical interaction between Rer1p and Sec71p. Lysates of the Δrer1 Δpep4 cells expressing Rer1-3HAp and Sec71p-GFP were prepared and let react with DSP. Cross-linked products were immunoprecipitated with the anti-GFP antibody, treated with β-mercaptoethanol to cleave the linker, and subjected to immunoblotting with anti-GFP and anti-HA antibodies. As shown in Figure 1c, Rer1-3HAp was reproducibly and dose-dependently cross-linked to Sec71p-GFP (lanes 3 and 7). This provides biochemical evidence that Sec71p is also a ligand of Rer1p, although its sequence and topology are completely different from Sec12p.
The ER Retrieval Signal of Sec71p Is Confined in the TMD
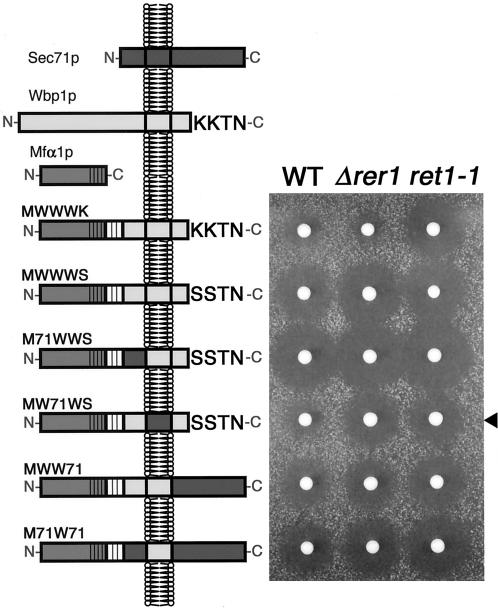
To identify and localize the Rer1p-dependent ER localization signal of Sec71p, we planned to construct chimeric proteins between Sec71p and an appropriate passenger protein that is innocent for its destination, as we did for the study of Sec12p (Sato et al., 1996). For this purpose, we first made a fusion protein between the α-mating factor precursor (Mfα1p) and the COOH-terminal half of Wbp1p (277–430 residues; see the left panel in Figure 2). Wbp1p is a type-I ER membrane protein, which functions as a component of the yeast N-oligosaccharyltransferase complex, and carries the KKXX signal at its COOH-terminus (te Heesen et al., 1991, 1992, 1993). The 3HA epitope was inserted between Mfα1p and Wbp1p. This chimeric protein named MWWWK was expressed in the wild-type, Δrer1, and a coatomer mutant ret1-1 cells. The ret1 mutant is known to be defective in the Golgi-to-ER retrieval of KKXX-harboring proteins including Wbp1p (Letourneur et al., 1994). The transformants were then examined for the secretion of α-factor using the halo assay (Nishikawa and Nakano, 1993; right panel, Figure 2). The halo, a growth inhibition zone around an α cell colony on the plate, indicates the area where the tester a cells spread as lawn is G0-arrested because of α-factor secreted from the colony. The diameter of the halo is proportional to the logarithm of the amount of α-factor and thus provides a quantitative measure of the secreted amount of α-factor. Because the processing of Mfα1p to yield mature α-factor takes place in the trans-Golgi, a large halo produced by Mfα1 chimera is an indication of its transport to the late Golgi. In the case of Wbp1p and Sec71p fusion proteins, ER localization is expected to lead to a small halo, whereas mistargeting to later compartments will give a large halo.
Figure 2.
The TMD of Sec71p contains a signal for ER localization. Various chimeras between Mfα1p, Wbp1p, and Sec71p were constructed as depicted on the left and expressed in the wild-type (SNY9), Δrer1 (SKY7), and ret1-1 (SKY27) cells. The transformants were examined for α-factor secretion by the halo assay. Note that a smaller halo indicates less secretion of α-factor, in other words, good ER localization of the chimeric protein. MW71WS (arrowhead) shows good ER localization in the wild-type but not in the Δrer1 or ret1-1 mutant.
As shown in Figure 2, MWWWK, which is localized to the ER by virtue of the KKXX motif, produced only a small amount of α-factor in the wild-type and Δrer1 mutant cells. The ret1-1 mutant made a large halo because it is defective in the KKXX protein retrieval. When the lysine residues in the KKXX motif were mutated to serines, the chimera produced a large halo even in the wild type (MWWWS). Now we performed a swapping experiment between MWWWS and Sec71p. Among several combinations we tested, only the MW71WS chimera, which contains the TMD from Sec71p, showed marked reduction of α-factor secretion (arrowhead). This effect was abolished in Δrer1. Thus, as was the case for Sec12p, the TMD region of Sec71p appears to contain the Rer1p-dependent ER retrieval signal.
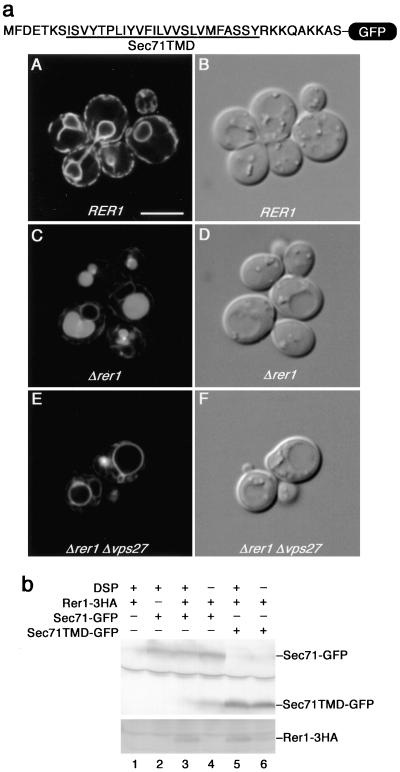
To corroborate this conclusion, we went on to construct a GFP chimera, which was fused to a small peptide fragment (35 residues) containing Sec71TMD (Figure 3a). This fusion protein (Sec71TMD-GFP) was introduced into the wild-type and Δrer1 cells and observed under the confocal laser scanning microscope (Figure 3a, A–D). In the wild-type cells, Sec71TMD-GFP was localized to the ER at steady state (Figure 3a, A), indicating that the TMD of Sec71p is sufficient for the insertion into the ER membrane and the ER residence. The ER localization was almost completely gone in Δrer1, indicating that it is clearly Rer1p dependent (Figure 3a, C). It should be noted that the GFP fluorescence was in the lumen of vacuoles in panel C, again suggesting the MVB sorting.
Figure 3.
The TMD of Sec71p is sufficient for interaction with Rer1p and for ER localization. (a) The Sec71TMD and its neighboring residues (35 amino acids) were fused to the NH2-terminus of GFP. The TMD region is underlined. This Sec71TMD-GFP fusion was expressed in the wild-type (SNY9), Δrer1 (SKY7), and Δrer1 Δvps27 (SKY80) cells and examined by confocal laser scanning microscopy. Fluorescence (A, C, and E) and Nomarski (B, D, and F) images are shown. (b) Physical interaction between Rer1p and the Sec71TMD. Lysates were prepared from Δrer1 Δpep4 cells (SKY42) coexpressing Rer1-3HAp on a multicopy plasmid and Sec71-GFP or Sec71TMD-GFP on a single-copy plasmid and incubated with or without DSP. The immunoprecipitates with the anti-GFP antibody were subjected to immunoblotting with anti-GFP and anti-HA antibodies.
We further asked whether Rer1p physically interacts with the Sec71TMD in this GFP construct (Figure 3b). In the presence of DSP, the cross-linking between Rer1-3HAp and the Sec71TMD-GFP was reproducibly detected (lane 5). These results demonstrate that the TMD of Sec71p does indeed function as the Rer1p-dependent ER localization signal.
To pursue the possibility that the mislocalized Sec71TMD-GFP (Figure 3a, C) was targeted to the vacuolar lumen via the MVB sorting pathway, we expressed this fusion in the Δrer1 Δvps27 double-mutant cells. Vps27p belongs to the class E Vps proteins and is required for MVB sorting. In the Δvps27 mutant, GFP-CPS is not transported to the vacuolar lumen but localized to the aberrant endosome-like structure (the class E compartment) and the vacuolar membrane (Odorizzi et al., 1998). As shown in Figure 3a, panel E, it was also the case for Sec71TMD-GFP. The mislocalized chimera in Δrer1 could not reach the lumen of vacuoles when the Δvps27 mutation was combined.
Spatial Location of Polar Residues in the Sec71TMD Is Important for the Recognition by Rer1p
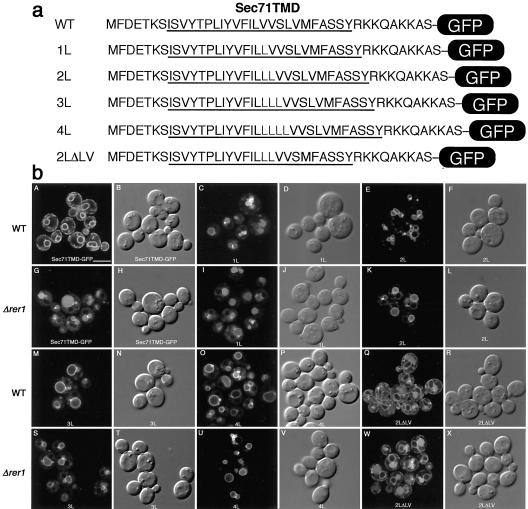
Having established that the Sec71TMD as well as the Sec12TMD contain Rer1p-dependent ER localization signals, we decided to determine the nature of this type of signal. By comparison of these two TMDs, we noticed a feature of hydrophobicity, which peaks in the middle of the TMD. In the Sec71TMD, there is a cluster of very hydrophobic amino acid residues VFILVV (see Figure 4, box). We will hereafter refer to this sequence as the highly hydrophobic region (HHR). We introduced a variety of mutations in this HHR and its flanking residues and examined their localization in the wild-type and rer1 cells by the halo assay (Figure 4). Expression of these mutants was comparable to that of the wild-type version as confirmed by immunoblotting (our unpublished results). We picked up eight independent transformants for each construct and quantified the amount of the secreted α-factor by measuring the radii of halos formed around them (Hopkins et al., 2000). To minimize the possible effect of ER retention caused by other mechanisms such as the ER quality control (Ellgaard et al., 1999), the Rer1p-dependent ER localization of each mutant protein was assessed by the ratio of the secreted α-factor from the wild-type cells to that from the Δrer1 cells (WT/Δrer1). Smaller numbers indicate better Rer1p-dependent ER localization. First, we introduced a polar residue into the HHR (LS) to examine whether the hydrophobicity of this region is critical for the Rer1p-dependent ER localization. This mutation did not significantly affect the Rer1p dependency. On the contrary, the replacement of the flanking polar residues (Y and S, asterisked on the top) by alanine or leucine (YL, YA, SL, and SA) resulted in marked increase of the α-factor secretion. Replacement to other polar residues (YS, YQ, and SQ) had more modest effects, suggesting that the presence of polar residues in these positions is very important for the recognition by Rer1p. We also inserted increasing numbers of leucine residues into the HHR and examined their effects. Interestingly, all the insertion mutants (1L, 2L, 3L, and 4L) lost the ability to remain in the ER. Deletion of the leucine from the HHR (ΔL) also caused a defect. It is not the whole length of the TMD that is important, because the 2LΔLV mutant, which had two leucines inserted in the HHR but downstream LV was deleted to keep the length of the TMD constant, was still defective in ER localization. These results indicate that the spatial distribution of the two polar residues that flank the HHR is the very important determinant for the Rer1p recognition.
Figure 4.
Mutational analysis of the Sec71TMD. In the MW71WS chimera of Figure 2, a variety of mutations were introduced into the TMD region. Putative TMDs are underlined. In the control (top), the highly hydrophobic region (HHR) and the flanking polar residues are marked with a box and asterisks, respectively. Sequences of the mutants constructed are shown below. These constructs were expressed in the wild-type (SNY9) and Δrer1 (SKY7) cells. Eight independent transformants were subjected to the halo assay and the secreted α-factor was quantified as described in MATERIALS AND METHODS. The ratio of the amount of secreted α-factor from the wild-type cells to that from the Δrer1 cells are shown on the right. Smaller numbers indicate better Rer1p-dependent ER localization.
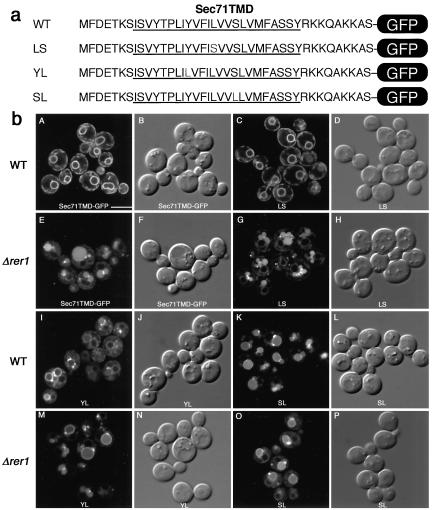
To visually confirm the effects of these mutations in the Sec71TMD, we constructed the same mutants in the Sec71TMD-GFP construct and observed under the confocal laser scanning microscope (Figures 5 and 6). Consistent with the results of the halo assay, the LS mutation in the HHR showed virtually no defect and complete ER localization in the Rer1p-dependent manner (Figure 5b). The mutations of the flanking polar residues (YL and SL) caused mislocalization of the GFP fusion to the vacuole. The GFP signals milocalized to vacuoles in the cases of YL and SL mutants are not completely lumenal, regardless of wild type or Δrer1. Significant portion is on the vacuolar membranes. This suggests that these polar residues are also involved in the MVB sorting late in the pathway. As shown in Figure 6b, all insertion mutants (1L, 2L, 3L, and 4L) were mislocalized to vacuoles in wild-type or Δrer1 cells, but the localization patterns in vacuoles were different. The GFP fluorescence of the 1L mutant was observed both on the membrane and in the lumen, but those of longer insertion mutants (2L–4L) were mostly on the vacuolar membrane. On the contrary, the 2LΔLV mutant, which shows a defect in the Rer1p-dependent ER localization, appears to be well sorted into the MVB pathway, suggesting that the length of TMD is important for MVB sorting. These results are similar to the previous findings about the MVB sorting of type-II membrane proteins and C-terminal tail-anchored proteins such as SNARE proteins (Reggiori et al., 2000). It appears that the Rer1p-dependent Golgi-to-ER retrieval and the MVB sorting in late endosomes both watch polar residues in the TMD, but the latter mechanism is more sensitive to the whole length of the TMD than the former.
Figure 5.
Localization of GFP-fused Sec71TMD mutants. (a) Amino acid sequences of the TMD mutants. Predicted TMD regions are underlined. (b) Effects of mutations of the polar residues. LS, YL, and SL mutants were expressed in the wild-type (SNY9) and Δrer1 (SKY7) cells and examined by confocal laser scanning microscopy. Fluorescence (left panel in the pair) and Nomarski (right panel in the pair) images are shown. Bar, 5 μm.
Figure 6.
Effects of the length of the TMD on the localization of Sec71TMD-GFP. Control and leucine insertion mutants (1L, 2L, 3L, 4L, and 2LΔLV; a) were expressed in the wild-type (SNY9) and Δrer1 (SKY7) cells and observed by confocal microscopy (b). Fluorescence (left panel in the pair) and Nomarski (right panel in the pair) images are shown. Bar, 5 μm.
Two Different Modes of Ligand recognition by Rer1p
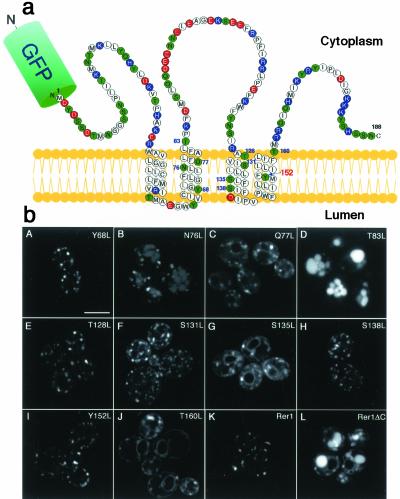
As a complementary approach to understanding the molecular mechanism of ligand-receptor interaction, we looked at the structural feature of Rer1p. Naturally, candidate residues of Rer1p that are involved in the recognition of the polar residues in Sec71TMD (as well as Sec12TMD) would be also present in the TMDs of Rer1p. Rer1p has four TMDs, which are unusually rich in polar residues (Figure 7a). We chose 10 noncharged polar residues in or near the TMDs (numbered in the diagram of Figure 7a), replaced each of them by leucine and examined their properties. First, the mutants in the context of GFP-Rer1p (Sato et al., 2001) were expressed in Δrer1 cells and observed by confocal microscopy. As shown in Figure 7b, Y68L, Q77L, T128L, S138L, and Y152L mutants stayed mostly in the Golgi at steady state. S131L, S135L, and T160L mutants gave mixed patterns of ER and Golgi. The T83L mutant was mislocalized to vacuoles, whereas the N76L mutant showed both Golgi and vacuolar localization.
Figure 7.
Mutational analysis on the Rer1p side. (a) Schematic structure of Rer1p. Polar residues are colored: red, acidic; blue, basic; green, noncharged. Ten noncharged polar amino acid residues in or near the four TMDs (shown numbered) were selected and mutated. (b) Subcellular localization of Rer1p mutants. The polar residues marked in panel a were individually mutated to leucine in the GFP-Rer1p construct and were analyzed for localization in Δrer1 cells (SKY7) by confocal microscopy. Rer1ΔC is the truncation mutant that lacks the COOH-terminal 25 residues. Bar, 5 μm.
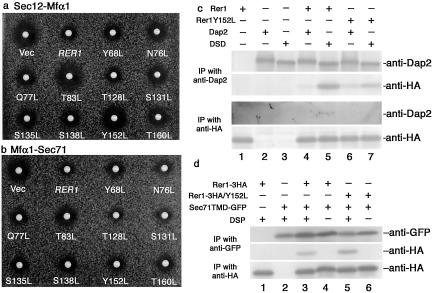
Next, the GFP-Rer1p mutants were coexpressed with either Sec12p-Mfα1p or Mfα1p-Sec71p in the Δrer1 cells, and their retrieval activities were tested by the halo assay (Figure 8, Table 2). The abilities to complement Δrer1 (smaller halo means better complementation) varied from mutant to mutant, and in general, mutants normally residing in the Golgi seemed to have better chances to function than others. The one exception, Y152L, appeared to recognize Mfα1-Sec71p very well (small halo in Figure 8b), but not Sec12-Mfα1p (large halo in Figure 8a). This result suggests the possibility that Tyr152 residue is differently involved in the recognition of Sec12p and Sec71p. So we further examined whether the Y152L mutant had indeed difference in the interaction with these two ligands. Figure 8, panels c and d, show the results of cross-linking experiments. Dap2p or dipeptidyl aminopeptidase B, a type-II vacuolar membrane protein (Suarez Rendueles and Wolf, 1987), has been used as a passenger protein to determine the ER localization signals of Sec12p (Sato et al., 1996). DSD is a chimeric protein comprised of the lumenal and cytoplasmic domains from Dap2p and the TMD from Sec12p and has shown to be almost completely localized to the ER by the Rer1p-dependent retrieval (Sato et al., 1996). As shown in Figure 8c, the Sec12TMD in the DSD chimera (see Sato et al., 1996) was recognized by and cross-linked with the wild-type Rer1p (IP with anti-Dap2 and immunoblot with anti-HA and vice versa). However, the efficiency of cross-link was much reduced when Rer1p Y152L was used (compare lanes 5 and 7). On the other hand, cross-linking between Sec71TMD and Rer1p (Figure 8d) was almost equally efficient for the wild-type Rer1p and the Y152L mutant (lanes 3 and 5). Thus the effect of the Y152L mutation was clearly different for Sec12p and Sec71p, suggesting distinct modes of interaction for Rer1p in the recognition of ligands with opposite topologies.
Figure 8.
Differential modes of interaction between Rer1p and its ligands. The Rer1pY152L mutant can recognize the Sec71TMD but not the Sec12TMD. (a and b) GFP-Rer1p and its TMD mutants complement Δrer1 to different degrees. The Δrer1 cells (SKY75) expressing either Sec12p-Mfα1p (a) or Mfα1p-Sec71p (b) were transformed with GFP-RER1 or its mutants. The transformants were examined for the α-factor secretion by the halo assay. Note that the Y152L mutant forms a large halo from Sec12-Mfα1p but not from Mfα1-Sec71p. (c) Reduced interaction between Rer1Y152L and the Sec12TMD. The Δdap2 cells (SMY22–10B) coexpressing Rer1-3HAp or Rer1Y152L-3HAp and Dap2p or DSD (Dap2-Sec12-Dap2 chimera) were subjected to cross-linking with DSP. The immunoprecipitates with anti-Dap2p or anti-HA antibodies were further analyzed by immunoblotting again with anti-Dap2p and anti-HA antibodies. (d) Rer1Y152L is normal for the interaction with the Sec71 TMD. Lysates were prepared from the Δrer1 Δpep4 cells (SKY42) coexpressing Sec71TMD-GFP and Rer1-3HAp or Rer1Y152L-3HA and incubated with or without DSP. The immunoprecipitates with the anti-GFP antibody were subjected to immunoblotting with anti-GFP or anti-HA antibodies.
Table 2.
Complementation activities of the Rer1TMD mutants for mislocalization of Sec12p-Mfα1p and Mfα1p-Sec71p in rer1
| Sec12p-Mfα1p | Mfα1p-Sec71p | |
|---|---|---|
| Vector | 100 ± 0 | 100 ± 0 |
| RER1 | 24 ± 3 | 14 ± 4 |
| Y68L | 32 ± 9 | 50 ± 18 |
| N76L | 48 ± 9 | 25 ± 3 |
| Q77L | 34 ± 8 | 16 ± 5 |
| T83L | 90 ± 11 | 74 ± 14 |
| T128L | 33 ± 7 | 21 ± 6 |
| S131L | 45 ± 5 | 49 ± 13 |
| S135L | 37 ± 4 | 18 ± 4 |
| S138L | 30 ± 3 | 16 ± 5 |
| Y152L | 56 ± 6 | 27 ± 6 |
| T160L | 41 ± 9 | 49 ± 13 |
Δrer1 cells (SKY75) expressing Sec12p-Mfα1p and rer1-2 cells (SKY5) expressing Mfα1p-Sec71p were transformed with vector alone, GFP-RER1, and the GFP-RER1 TMD mutants indicated. Four independent transformants were subjected to the halo assay, and the secreted α-factor was quantified. The amounts of secreted α-factor are shown as proportions relative to that secreted from the Δrer1 cells carrying vector alone (100%). Smaller numbers indicate better ER localization.
DISCUSSION
The question as to how membrane proteins are sorted from each other during dynamic membrane traffic is one of the central issues of organelle identification. Interactions through TMDs have been thought to be the key for mutual recognition but its molecular details remained elusive. In the present study, we have shown that the presence of polar residues in the TMD and the whole length of the TMD are used as independent cues of such recognition.
ER Localization of Membrane Proteins
In addition to the COOH-terminal KDEL/HDEL (Munro and Pelham, 1987; Pelham, 1988) and KKXX (Jackson et al., 1990) signals, TMDs have been known to serve as ER localization determinants (Pedrazzini et al., 1996; Sato et al., 1996; Rayner and Pelham, 1997; Honsho et al., 1998). Rer1p is a sorting receptor in the Golgi apparatus to retrieve a set of ER membrane proteins from Golgi to ER (Nishikawa and Nakano, 1993; Sato et al., 1997; Massaad et al., 1999). It physically recognizes the TMDs of Sec12p (Sato et al., 2001) and Sec71p (this study; Figures 1,2,3) and thus provides an ideal system to understand the mechanism of membrane protein sorting.
Our detailed mutational analysis of the Sec71TMD demonstrated that the essential feature to be recognized by Rer1p is the presence of two polar amino acid residues (Y and S) that flank the very hydrophobic cluster VFILVV (HHR; Figures 4,5,6). The distance of these polar residues is important because insertion or deletion of leucine residues into and from the HHR almost completely abolished the ability to act as the retrieval signal. A very similar motif is seen in the TMD of Sec12p. In this case, two sets of polar residues TN and SY stand on the edges of the HHR, FILIVLL in this case, which motif is also proved essential for the Rer1p-dependent ER localization (Sato et al., 1996, 2001). It should be reminded that Sec12p and Sec71p are both transmembrane proteins with a single TMD, but in the opposite topology. Nevertheless, the structural feature that polar residues flank the HHR is common. The length of the HHR is a little different: seven residues for Sec12p and six for Sec71p. These numbers correspond to about two turns if α-helix conformation is assumed, but considering their opposite directions, the binding modes cannot be the same.
Another possible feature of TMDs that could be recognized during sorting is the length. For Sec71p to be recognized by Rer1p for ER retrieval, the TMD length itself appears not to be important. The key experiment was the use of the 2LΔLV mutant, in which two leucines were inserted into the HHR while downstream LV was removed to keep the length constant (Figure 4). This mutant form was not recognized by Rer1p any more, indicating that it is the distance of the two polar residues but not the whole length of the TMD that Rer1p is watching. A small part of Sec71TMD-GFP 2LΔLV still localizes to the ER even in the Δrer1 mutant (Figure 6b, panel w), suggesting that there are Rer1p-independent ER localization mechanism(s) as well (Rayner and Pelham, 1997).
Coming back to the difference between Sec12p and Sec71p, a very important insight into the binding modes of Rer1p was provided by the mutational analysis of the TMDs of Rer1p (Figures 7 and 8). Among the mutants we constructed, Y152L showed a very interesting behavior. The Y152L mutant Rer1p had a problem in the recognition of Sec12p but not Sec71p. This selective defect for Sec12p was further confirmed by a cross-linking experiment. Thus the Tyr152 residue in the fourth TMD of Rer1p is important for the binding mode of Sec12p but not for that of Sec71p. This residue is completely conserved among the known members of the Rer1p family (Sato et al., 1999). Presumably, in order to accommodate a variety of ER membrane proteins for the Rer1p-dependent retrieval system, Rer1p would be capable of taking several different modes of binding for different ligands. The ultimate proof of this hypothesis will await structural analysis of the receptor-ligand complex, which will be a very challenging but fruitful project in the future.
Sorting of Membrane Proteins into Multivesicular Bodies
In the process of analyzing the structural requirements of the Sec71TMD to be retrieved by Rer1p, we found that the motif of Sec71TMD could also be recognized by the MVB sorting mechanism. In the Δrer1 mutant cells, Sec71p is not efficiently localized to the ER and the majority is missorted to the lumen of vacuoles (Figures 1 and 3). This was indeed due to the MVB sorting because the knockout of VPS27, a gene required in this pathway, abolished targeting of Sec71p-GFP to the vacuolar lumen.
Sec71p is a type-III membrane protein, of which N-terminus is facing the ER lumen and the C termini is cytoplasmic (Feldheim et al., 1993, Kurihara and Silver, 1993). In the case of type-II transmembrane proteins such as SNARE proteins, the presence of a polar residue in the TMD at the position of third or fifth from the cytoplasmic border and the whole length of TMD are both crucial for the MVB targeting (Reggiori and Pelham, 2000). The mutational analysis of Sec71TMD-GFP revealed that the polar residues essential for the Golgi-to-ER retrieval by Rer1p are also important for the MVB sorting. When these residues were mutated, fluorescence from the limiting membrane of vacuoles became clearer, indicating that these mutants were less efficiently sequestrated into lumenal vesicles (Figure 5). The distance between these two polar residues first appeared to be again important because leucine insertion/deletion mutants in the HHR were also defective in efficient MVB sorting. However, in the case of the 2LΔLV mutant, the mutant Sec71TMD-GFP was mostly missorted to the vacuole due to the disability to be recognized by Rer1p as discussed above, but was still targeted to the vacuolar lumen. In other words, this mutant TMD is not a good ligand for Rer1p but is recognized by the MVB-sorting mechanism. Because the 2LΔLV mutant contains the two polar residues in a different arrangement from the wild type but has the same length of TMD, these results imply that the MVB-sorting machinery senses the length of the type-III TMD strictly but not the positions of polar residues very rigorously unlike the cases of type-II membrane proteins and C-terminal tail-anchored proteins. These observations suggest that a similar but different mechanism is responsible for the MVB sorting of membrane proteins.
A Concept of Sorting Chaperones
The exposure of polar amino acid residues in the lipid bilayer would be a dangerous event. The fact that the two independent sorting mechanisms, Rer1p-dependent ER retrieval and the MVB sorting in late endosomes, recognize polar residues as the cue may indicate that these processes are developed to avoid deleterious effects of polar TMDs (see also Reggiori and Pelham, 2002). In fact, the MVB sorting leads to the dead end in the vacuole, i.e., destruction, and the ER retrieval could result in the quality control in the ER.
For the case of Rer1p, one of the biggest questions that remain to be answered in the future is how it releases the ligand in the ER. The ligands of Rer1p so far identified appear to have characteristics to form oligomers in their normal status. Sec71p and Sec63p are known to be in the posttranslational translocon complex and Sec12p may well be in oligomers in the ER. It could be the monomeric form of these molecules that are recognized by Rer1p in the Golgi. Higher affinity with its native partner(s) would explain the force to dissociate the ligand from Rer1p in the ER. If this hypothesis is correct, the role of Rer1p in the Golgi would be not only as the receptor to catch mistransported proteins but also as a chaperone to conceal polar residues exposed in the lipid bilayer. Analysis of their oligomerization states will be very important to address this problem. In this context, it is interesting that some membrane proteins are recognized by Rer1p only when the TMD was mutated. Such examples can be seen for invertase-Gas1p and Ste2p (Letourneur and Cosson, 1998). Whether the same scenario also applies to these cases would be a curious question.
In mammalian cells, unassembled chains of the T-cell antigen receptor (TCR) complex are known to be often localized to the ER and subsequently degraded by the ER-associated degradation (Klausner and Sitia, 1990). In the case of the TCR α chain, two positively charged amino acid residues in the TMD are critical for retention and ER degradation (Bonifacino et al., 1990a). Introduction of a polar residue in the TMD of the Tac antigen has also been shown to convert its destination from the plasma membrane to the ER and degradation (Bonifacino et al., 1991). These observations have been regarded as good examples of the quality control in the ER but for us they appear to point to the putative role of Rer1p-dependent retrieval in these processes. Whether the retrieval by Rer1p indeed plays a role in the ER quality control can be tested in yeast and is now being pursued.
Acknowledgments
We are grateful to Randy Schekman, Hugh Pelham, and Kyohei Umebayashi for strains; to Yoshihiro Amaya and Hiroshi Abe for anti-Dap2p and anti-GFP antibodies; and to the members of the Nakano laboratory for helpful comments and discussions. This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan, by grants from the Biodesign and Bioarchitect Research Projects of Riken, and by a President's Special Research Grant of Riken.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–12–0777. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-12-0777.
Abbreviations used: CCD, charge coupled device; COP, coat protein; CPS, carboxypeptidase S; DSP, dithiobis(succinimidyl propionate); GFP, green fluorescent protein; HHR, highly hydrophobic region; MVB, multivesicular body; ORF, open reading frame; TMD, transmembrane domain.
References
- Boehm, J., Ulrich, H.D., Ossig, R., and Schmitt, H.D. (1994). Kex2-dependent invertase secretion as a tool to study the targeting of transmembrane proteins which are involved in ER→ Golgi transport in yeast. EMBO J. 13, 3696–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino, J.S., Cosson, P., and Klausner, R.D. (1990a). Colocalized transmembrane determinants for ER degradation and subunit assembly explain the intracellular fate of TCR chains. Cell 63, 503–513. [DOI] [PubMed] [Google Scholar]
- Bonifacino, J.S., Cosson, P., Shah, N., and Klausner, R.D. (1991). Role of potentially charged transmembrane residues in targeting proteins for retention and degradation within the endoplasmic reticulum. EMBO J. 10, 2783–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino, J.S., Suzuki, C.K., and Klausner, R.D. (1990b). A peptide sequence confers retention and rapid degradation in the endoplasmic reticulum. Science 247, 79–82. [DOI] [PubMed] [Google Scholar]
- Camirand, A., Heysen, A., Grondin, B., and Herscovics, A. (1991). Glycoprotein biosynthesis in Saccharomyces cerevisiae. Isolation and characterization of the gene encoding a specific processing α-mannosidase. J. Biol. Chem. 266, 15120–15127. [PubMed] [Google Scholar]
- Deshaies, R.J., Sanders, S.L., Feldheim, D.A., and Schekman, R. (1991). Yeast Sec proteins involved in translocation into the endoplasmic reticulum into a membrane-bound multisubunit complex. Nature 349, 806–807. [DOI] [PubMed] [Google Scholar]
- Ellgaard, L., Molinali, M., and Helenius, A. (1999). Setting the standards: quality control in the secretory pathway. Science 286, 1882–1888. [DOI] [PubMed] [Google Scholar]
- Feldheim, D., Yoshimura, K., Admon, A., and Schekman, R. (1993). Structural and functional characterization of Sec66p, a new subunit of the polypeptide translocation apparatus in the yeast endoplasmic reticulum. Mol. Biol. Cell 4, 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick, K.G., Boothroyd, J.C., Rudner, A.D., and Pelham, H.R. (1992). Genes that allow yeast cells to grow in the absence of the HDEL receptor. EMBO J. 11, 4187–4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honsho, M., Mitoma, J.Y., and Ito, A. (1998). Retention of cytochrome b5 in the endoplasmic reticulum is transmembrane and luminal domain-dependent. J. Biol. Chem. 273, 20860–20866. [DOI] [PubMed] [Google Scholar]
- Hopkins, B.D., Sato, K., Nakano, A., and Graham, T.R. (2000). Introduction of Kex2 cleavage sites in fusion proteins for monitoring localization and transport in yeast secretory pathway. Methods Enzymol. 327, 107–118. [DOI] [PubMed] [Google Scholar]
- Jackson, M.R., Nillson, T., and Peterson, P.A. (1990). Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J. 9, 3153–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner, R.D., and Sitia, R. (1990). Protein degradation in the endoplasmic reticulum. Cell 62, 611–614. [DOI] [PubMed] [Google Scholar]
- Kurihara, T., and Silver, P. (1993). Suppression of a sec63 mutation identifies a novel component of the yeast endoplasmic reticulum translocation apparatus. Mol. Biol. Cell 4, 919–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur, F., and Cosson, P. (1998). Targeting to the endoplasmic reticulum in yeast cells by determinants present in transmembrane domains. J. Biol. Chem. 273, 33273–33278. [DOI] [PubMed] [Google Scholar]
- Letourneur, F., Gaynor, E.C., Hennecke, S., Démollière, C., Duden, R., Emr, S.D., Riezman, H., and Cosson, P. (1994). Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell 79, 1199–1207. [DOI] [PubMed] [Google Scholar]
- Lewis, M.J., Nichols, B.J., Prescianotto-Baschong, C., Riezman, H., and Pelham, H.R. (2000). Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes. Mol. Biol. Cell 11, 23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaad, M.J., Franzusoff, A., and Herscovics, A. (1999). The processing α1, 2-mannosidase of Saccharomyces cerevisiae depends on Rer1p for its localization in the endoplasmic reticulum. Eur. J. Cell Biol. 78, 435–440. [DOI] [PubMed] [Google Scholar]
- Munro, S. (1995). An investigation of the role of transmembrane domains in Golgi protein retention. EMBO J. 14, 4695–4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro, S., and Pelham, H.R. (1987). A C-terminal signal prevents secretion of luminal ER proteins. Cell 48, 899–907. [DOI] [PubMed] [Google Scholar]
- Nishikawa, S., and Nakano, A. (1993). Identification of a gene required for membrane protein retention in the early secretory pathway. Proc. Natl. Acad. Sci. USA 90, 8179–8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi, G., Babst, M., and Emr, S.D. (1998). Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell 95, 847–858. [DOI] [PubMed] [Google Scholar]
- Pedrazzini, E., Villa, A., and Borgese, N. (1996). A mutant cytochrome b5 with a lengthened membrane anchor escapes from the endoplasmic reticulum and reaches the plasma membrane. Proc. Natl. Acad. Sci. USA 93, 4207–4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrazzini, E., Villa, A., Longhi, R., Bulbarelli, A., and Borgese, N. (2000). Mechanism of residence of cytochrome b(5), a tail-anchored protein, in the endoplasmic reticulum. J. Cell Biol. 148, 899–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham, H.R.B. (1988). Evidence that luminal ER proteins are sorted from secreted proteins in a post-ER compartment. EMBO J. 7, 913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner, J.C., and Pelham, H.R.B. (1997). Transmembrane domain-dependent sorting of proteins to the ER and plasma membrane in yeast. EMBO J. 16, 1832–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori, F., Black, M.W., and Pelham, H.R. (2000). Polar transmembrane domains target proteins to the interior of the yeast vacuole. Mol. Biol. Cell 11, 3737–3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori, F., and Pelham, H. (2001). Sorting of proteins into multivesicular bodies; ubiquitin-dependent and -independent targeting. EMBO J. 20, 5176–5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori, F., and Pelham, H. (2002). A transmembrane ubiquitin ligase required to sort membrane proteins into multivesicular bodies. Nat. Cell Biol. 4, 117–123. [DOI] [PubMed] [Google Scholar]
- Rothblatt, J.A., Deshaies, R.J., Sanders, S.L., Daum, G., and Schekman, R. (1989). Multiple genes are required for proper insertion of secretory proteins into the endoplasmic reticulum in yeast. J. Cell Biol. 109, 2641–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler, I., Chiang, A., Kurihara, T., Rothblatt, J.A., Way, J., and Silver, P. (1989). A yeast gene important for protein assembly into the endoplasmic reticulum and the nucleus has homology to DnaJ, an Escherichia coli heat shock protein. J. Cell Biol. 109, 2665–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, K., Nishikawa, S., and Nakano, A. (1995). Membrane protein retrieval from the Golgi apparatus to the endoplasmic reticulum (ER): characterization of the RER1 gene products as a component involved in ER localization of Sec12p. Mol. Biol. Cell 6, 1459–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, K., Sato, M., and Nakano, A. (1997). Rer1p as common machinery for the endoplasmic reticulum localization of membrane proteins. Proc. Natl. Acad. Sci. USA 94, 9693–9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, K., Sato, M., and Nakano, A. (2001). Rer1p, a retrieval receptor for endoplasmic reticulum membrane proteins, is dynamically localized to the Golgi apparatus by coatomer. J. Cell Biol. 152, 935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, K., Ueda, T., and Nakano, A. (1999). The Arabidopsis thaliana RER1 gene family: its potential role in the endoplasmic reticulum localization of membrane proteins. Plant Mol. Biol. 41, 815–824. [DOI] [PubMed] [Google Scholar]
- Sato, M., Sato, K., and Nakano, A. (1996). Endoplasmic reticulum localization of Sec12p is achieved by two mechanisms: Rer1p-dependent retrieval that requires the transmembrane domain and Rer1p-independent retention that involves the cytoplasmic domain. J. Cell Biol. 134, 279–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele, P., Roth, M.G., and Simons, K. (1997). Interaction of influenza virus haemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO J. 16, 5501–5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski, R.S., and Hieter, P. (1989). A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez Rendueles, P., and Wolf, D.H. (1987). Identification of the structural gene for dipeptidyl aminopeptidase yscV (DAP2) of Saccharomyces cerevisiae. J. Bacteriol. 169, 4041–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Heesen, S., Janetzky, B., Lehle, L., and Aebi, M. (1992). The yeast WBP1 is essential for oligosaccharyltransferase activity in vivo and in vitro. EMBO J. 11, 2071–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Heesen, S., Knauer, R., Lehle, L., and Aebi, M. (1993). Yeast Wbp1p and Swp1p form a protein complex essential for oligosaccharyltransferase activity. EMBO J. 12, 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Heesen, S., Rauhut, R., Aebersold, R., Abelson, J., Aebi, M., and Clark, M.W. (1991). An essential 45 kDa yeast transmembrane protein reacts with anti-nuclear pore antibodies: purification of the protein, immunolocalization and cloning of the gene. Eur. J. Cell Biol. 56, 8–18. [PubMed] [Google Scholar]
- Zaliauskiene, L., Kang, S., Brouillette, C.G., Lebowitz, J., Arani, R.B., and Collawn, J.F. (2000). Down-regulation of cell surface receptors is modulated by polar residues within the transmembrane domain. Mol. Biol. Cell 11, 2643–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]