Abstract
Class I chitinase (Chi9) and β-1,3-glucanase (GluB) genes are expressed in the micropylar endosperm cap of tomato (Lycopersicon esculentum) seeds just before radicle emergence through this tissue to complete germination. In gibberellin (GA)-deficient mutant (gib-1) seeds, expression of Chi9 and GluB mRNA and protein is dependent upon GA. However, as expression occurs relatively late in the germination process, we investigated whether the genes are induced indirectly in response to tissue wounding associated with endosperm cap weakening and radicle protrusion. Wounding and methyl jasmonate (MeJA) induced Chi9 expression, whereas ethylene, abscisic acid, sodium salicylate, fusicoccin, or β-aminobutyric acid were without effect. Chi9 expression occurred only in the micropylar tissues when seeds were exposed to MeJA or were wounded at the chalazal end of the seed. Expression of Chi9, but not GluB, mRNA was reduced in germinating seeds of the jasmonate-deficient defenseless1 tomato mutant and could be restored by MeJA treatment. Chi9 expression during germination may be associated with “wounding” from cell wall hydrolysis and weakening in the endosperm cap leading to radicle protrusion, and jasmonate is involved in the signaling pathway for this response. Among these treatments and chemicals (other than GA), only MeJA and wounding induced a low level of GluB expression in gib-1 seeds. However, MeJA, wounding, and particularly ethylene induced both genes in leaves, whereas GA induced only Chi9 in leaves. Although normally expressed simultaneously during tomato seed germination, Chi9 and GluB genes are regulated distinctly and tissue specifically by hormones and wounding.
In seeds whose embryos are embedded in endosperm tissues, such as tomato (Lycopersicon esculentum; Groot and Karssen, 1987), the tissues surrounding the embryo can act as a mechanical barrier to radicle emergence (Bewley, 1997). The expansive force from the embryo axis must exceed the mechanical restraint of the tissues enclosing the radicle tip for germination to be completed. Therefore, germination is controlled by the balance between the physical resistance of the enclosing tissues and the growth force of the embryo after imbibition (Ni and Bradford, 1992). Because mechanical restraint from the endosperm cap is the major factor limiting tomato seed germination, weakening of the micropylar endosperm tissue is a prerequisite for radicle emergence (Groot and Karssen, 1987). Cell wall hydrolases are expected to be involved in micropylar endosperm weakening and germination, and a number of hydrolases/wall proteins are expressed in the cap in association with germination, including endo-β-mannanase, α-mannosidase, α-galactosidase, polygalacturonase, xyloglucan endotransglycosylase, peroxidase, β-1,3-glucanase, chitinase, and expansin (Sitrit et al., 1999; Chen and Bradford, 2000; Nonogaki et al., 2000; Chen et al., 2002; Feurtado et al., 2001; Mo and Bewley, 2002; Morohashi, 2002).
Leubner-Metzger et al. (1995, 1996) showed that class I β-1,3-glucanase (EC 3.2.1.39) mRNA accumulation, enzyme activity, and protein content increased specifically in the endosperm cap tissue of tobacco (Nicotiana tabacum) seeds just before radicle emergence. Furthermore, in contrast to the usual inhibitory effect of abscisic acid (ABA), both β-1,3-glucanase expression and endosperm rupture were promoted by ABA in transgenic seeds containing a chimeric ABA-inducible β-1,3-glucanase transgene (Leubner-Metzger and Meins, 2000). The absence of significant accumulation of chitinase (EC 3.2.1.14), which is usually coexpressed with β-1,3-glucanase in pathogenesis-related responses (van Loon, 1997), and the close relationship between β-1,3-glucanase expression and completion of germination, led to the hypothesis that β-1,3-glucanase contributed to the hydrolysis of cell wall components resulting in endosperm weakening at the site of radicle protrusion from tobacco seeds (Leubner-Metzger et al., 1995, 1996; Leubner-Metzger and Meins, 2000; Leubner-Metzger, 2003).
In contrast to tobacco seeds, we previously reported that class I β-1,3-glucanase (GluB) and chitinase (Chi9) genes are expressed in germinating tomato seeds (Wu et al., 2001). The mRNAs, proteins, and enzymatic activities were detected specifically in the micropylar tissues before radicle emergence. The close correlation between tomato seed germination and accumulation of these two hydrolases was further shown in gibberellin (GA)-deficient (gib-1) mutant tomato seeds, which do not complete germination unless exogenous GA is provided (Groot and Karssen, 1987). Chi9 and GluB expression was detected in gib-1 tomato seeds only when supplied with GA. ABA, which delays or prevents radicle emergence but not endosperm weakening, reduced the abundance of GluB mRNA, protein, and enzyme activity during wild-type cv Moneymaker (MM) tomato seed germination, but had no effect on expression of Chi9. We tested the possibility that these enzymes are involved in endosperm cap cell wall hydrolysis and weakening, but found no evidence that they were directly involved in these processes in tomato (Wu et al., 2001). Therefore, the reason for their joint expression in the micropylar tissue of tomato seeds just before radicle emergence remains unknown.
Chitinases and β-1,3-glucanases, generally encoded by multigenic families, are widely distributed in the plant kingdom and have diverse roles in plant growth and development, including microsporogenesis, embryogenesis, germination, flowering, and abscission, as well as in wounding and defense responses (for review, see Meins et al., 1992; Leubner-Metzger and Meins, 1999; Neuhaus, 1999; Gomez et al., 2002; Leubner-Metzger, 2003). Plant chitinases and β-1,3-glucanases have been classified by sequence similarity into six and four families, respectively. As noted above, only class I genes (Chi9 and GluB), which contain vacuolar-targeting signals (van Kan et al., 1992; Danhash et al., 1993), were expressed in germinating tomato seeds (Wu et al., 2001). β-1,3-Glucanases and chitinases are well-known pathogenesis-related proteins that are constitutively expressed at low levels in plants, but are dramatically induced when plants respond to infection by fungal, bacterial, or viral pathogens (Leubner-Metzger and Meins, 1999; Neuhaus, 1999; van Loon, 1999). Several in vitro experiments have demonstrated that chitinases and β-1,3-glucanases were able to partially degrade the cell walls and inhibit mycelial growth or spore germination of certain pathogenic fungi, and the antifungal effects were synergistically enhanced when both enzymes were present (e.g. Sela-Buurlage et al., 1993; Lawrence et al., 1996; Anfoka and Buchenauer, 1997). The constitutive expression of chitinase and/or β-1,3-glucanase genes in transgenic plants provided further evidence for their roles in plant defense against certain fungal pathogens, especially when both were expressed simultaneously (Meins et al., 1992; Stintzi et al., 1993; Jongedijk et al., 1995). Furthermore, chitinase and β-1,3-glucanase can also work indirectly by releasing oligosaccharides that can act as elicitors to activate other plant defense responses (Shibuya and Minami, 2001).
Consistent with a role in plant defense, β-1,3-glucanase and chitinase genes often are expressed in response to wounding (Zhou, 1999). Putative wounding signals in plants include chemical compounds such as ethylene, salicylic acid (SA), ABA, jasmonic acid (JA) or methyl jasmonic acid (MeJA), and systemin (Leon et al., 2001), as well as bioelectrical (Peña-Cortés and Willmitzer, 1995) and hydraulic signals (Malone and Alarcon, 1995). JA and its methyl ester (MeJA), two cyclopentanone compounds, are key components of a wound signal transduction cascade in plants. For example, the application of exogenous JA induces the expression of a variety of genes, such as Phe ammonia lyase and proteinase inhibitors, that are also responsive to wounding (Howe et al., 1996; McConn et al., 1997). Using tomato JA biosynthesis and sensitivity mutants in grafting experiments, Howe and coworkers (Li et al., 2002; Lee and Howe, 2003) further demonstrated that JA or a derivative of it may also act as a long-distance transmissible wound signal. In tomato leaves, β-1,3-glucanases and chitinases can be induced by MeJA (Chao et al., 1999), SA and its analogs (2,6-dichloroisonicotinic acid and benzo-[1,2,3]-thiodiazole-7-carbothionic acid S-methyl ester; van Kan et al., 1995), ethylene (van Kan et al., 1995; Chao et al., 1999), β-aminobutyric acid (BABA; Cohen et al., 1994), and fusicoccin (FC; Roberts and Bowles, 1999; Schaller et al., 2000). Morohashi and Matsushima (2000) reported that wounding by bisecting imbibed tomato seeds stimulated β-1,3-glucanase (but not chitinase) activity in the lateral endosperm and proposed that β-1,3-glucanase expression during tomato seed germination could be a response to wounding caused by the penetration of the radicle through the endosperm cap tissue.
We have further investigated the possible role of wounding and other stimuli in regulating Chi9 and GluB expression during tomato seed germination. Here, we report that wounding or MeJA treatment induced Chi9 (but not GluB) expression specifically in the micropylar tissue of gib-1 tomato seeds in the absence of GA. Interestingly, Chi9 expression occurred only in the micropylar tissues even when other parts of the seed were wounded. We also show that the regulation of Chi9 and GluB in seeds is distinct from that in leaves.
RESULTS
Wounding Induces Chi9 mRNA Accumulation in the Micropylar Region of gib-1 Seeds
Chi9 and GluB are the only expressed isoforms of chitinase and β-1,3-glucanase, respectively, during germination of wild-type (MM) tomato seeds, and both were induced in gib-1 tomato seeds only when GA was supplied (Wu et al., 2001). Therefore, we used water-imbibed gib-1 seeds to test whether Chi9 and GluB expression was responsive to wounding or different elicitors in the absence of GA. We demonstrated previously that the accumulation of mRNA of these genes was invariably accompanied by appearance of the corresponding protein and enzymatic activity, and that mRNA accumulation could be assayed directly in individual seeds by tissue printing (Wu et al., 2001). Therefore, we used tissue printing to screen for the effects of various wounding and chemical treatments on expression and localization of Chi9 and GluB in gib-1 tomato seeds.
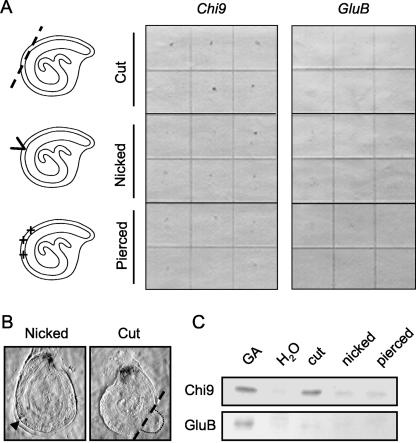
When a small piece of lateral endosperm at the chalazal end of the imbibed seed was removed (cut treatment), Chi9 expression was induced in the endosperm cap tissues, but not in the tissues adjacent to the wound site (Fig. 1, A and B). However, because the embryo protruded hypocotyl-first through the cut site (Fig. 1B), Chi9 expression could be a response to embryo growth rather than specifically to wounding. To test the effect of wounding per se in the absence of embryo growth, we nicked or pierced each seed in the lateral endosperm at the chalazal end using a dissecting blade or needle, respectively. Both of these treatments induced Chi9 mRNA appearance specifically in the endosperm cap tissue 36 h after wounding, although at lower abundance than after the cut treatment (Fig. 1, A and B). Chi9 protein also accumulated in micropylar tissues after cutting and in lesser amounts after nicking or piercing (Fig. 1C), in agreement with the tissue print results. In contrast, GluB mRNA and protein expression were not detected in the same experiments (Fig. 1, A and C). Therefore, wounding intact gib-1 seeds in the absence of GA can induce Chi9, but not GluB, expression and, interestingly, the response was exhibited only in the micropylar tissue regardless of where the seed was wounded (Fig. 1, A and B).
Figure 1.
The effect of wounding on accumulation of tomato Chi9 and GluB mRNA and proteins in imbibed GA-deficient gib-1 tomato seeds. A, Tissue printing was used to detect and localize Chi9 and GluB mRNA expression in individual gib-1 seeds. After 12 h of imbibition in water at 25°C, each gib-1 seed was wounded on the endosperm of its chalazal end by cutting off a piece (cut treatment), by nicking without removing tissue (nicked treatment), or by piercing with a pin (pierced treatment). After further incubation in the same conditions for 36 h, individual seeds were bisected and the cut surface of each one-half was printed directly on separate nylon membranes. The mirror-image tissue prints were then hybridized with antisense riboprobes for Chi9 (left) or GluB (right). Six replicate prints are shown for each treatment. B, Magnified tissue print images of nicked (left) and cut (right) gib-1 tomato seeds hybridized with antisense Chi9 riboprobe. The arrowhead on the left panel points to the nick site on the tomato seed. The dashed line in the right panel marks where the lateral endosperm was cut and the dotted line outlines the protruding hypocotyl of the embryo. Note the hybridization signal in the micropylar endosperm and the absence of signal at the wound sites. C, Immunoblot analyses of tomato Chi9 and GluB proteins in response to wounding treatments. The seeds were incubated and wounded as described above, and intact seeds were incubated in 100 μm GA4+7 as a positive control. The micropylar tissues of the seeds were dissected 36 h after wounding and proteins were extracted. Equal amounts of protein (50 μg) were loaded in each lane. Western blots of tomato seed proteins are shown using antiserum against tobacco class I chitinase (top) and β-1,3-glucanase (bottom), respectively (Wu et al., 2001).
Ethylene and ABA Are Not Required for Chi9 and GluB Expression during Tomato Seed Germination
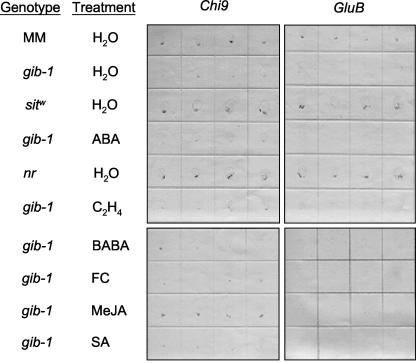
Several lines of evidence indicate that ethylene and ABA are components in the signal transduction pathway of the systemic wounding response in tomato plants (Peña-Cortés and Willmitzer, 1995), and that the ABA and ethylene signaling pathways can interact to regulate germination (Beaudoin et al., 2000; Ghassemian et al., 2000). Therefore, we investigated whether these two phytohormones are required for Chi9 and/or GluB expression during tomato seed germination by using seeds of Never ripe (nr), an ethylene-insensitive mutant, and of sitw, an ABA-deficient mutant. The Nr gene encodes an ethylene receptor protein that when mutated, as in homozygous nr plants, results in insensitivity to ethylene at all stages of development tested (Klee and Tieman, 2002). The Sitw gene encodes an abscisic aldehyde oxidase gene that when mutated (sitw) results in reduced ABA content in plants and seeds (Groot and Karssen, 1992; Schwartz et al., 2003). Chi9 and GluB mRNA were expressed in nr and sitw tomato seeds imbibed in water (Fig. 2), and seeds of both mutants germinated normally. Exposure to 10 μL L-1 ethylene or 100 μm ABA for 48 h did not cause significant accumulation of GluB or Chi9 transcripts in gib-1 seeds (Fig. 2; similar data for 1 and 100 μL L-1 ethylene not shown). We conclude that ethylene and ABA are not required for expression of Chi9 and GluB in germinating tomato seeds, and they cannot substitute for GA in inducing expression of these genes.
Figure 2.
Tissue print analyses of tomato Chi9 and GluB mRNA expression in seeds of tomato hormone response or synthesis mutants and in response various elicitors. Chi9 (left panels) and GluB (right panels) mRNAs were assayed in seeds of the tomato genotypes indicated (wild-type, cv MM; GA-deficient mutant, gib-1; ethylene-insensitive mutant, nr; ABA-deficient mutant, sitw) after imbibition for 48 h at 25°C in the indicated treatment solution (water; 100 μm ABA; 10 μL L-1 ethylene, C2H4; 19 mm BABA; 10 μm FC; 100 μm MeJA; or 1 mm SA). Wild-type cv MM and gib-1 seeds served as positive and negative controls, respectively, for both genes. Prints of gib-1 seeds imbibed in GA are identical to those for cv MM seeds (data not shown). Four replicate tissue prints (as described in Fig. 1) are shown for each genotype or treatment.
MeJA Induces Chi9 Transcript Accumulation in the Micropylar Endosperm of gib-1 Tomato Seeds
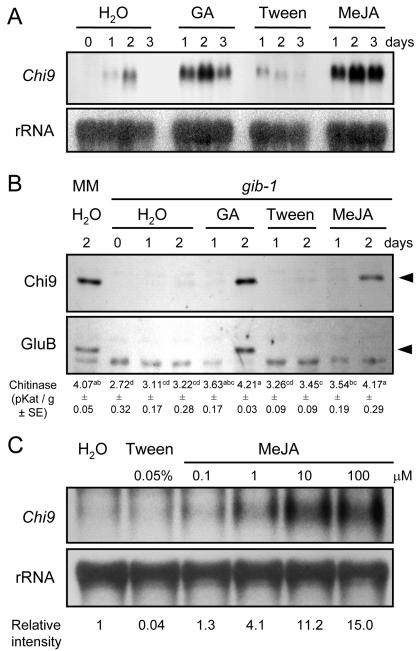
Other elicitor chemicals were tested to determine whether any of them was capable of stimulating Chi9 and/or GluB expression in gib-1 seeds. No significant accumulations of these mRNAs were detected in gib-1 seeds after a 48-h incubation in SA (0.1, 1, 10, and 20 mm), FC (0.1, 1, 10, and 100 μm), or BABA (10, 19, 29, and 48 mm; examples shown in Fig. 2). In contrast, 100 μm MeJA consistently stimulated Chi9, but not GluB, expression in gib-1 seeds (Fig. 2). As occurred after wounding, Chi9 mRNA was localized in the micropylar tissue. Northern blots confirmed that MeJA-treated gib-1 seeds showed a strong Chi9 mRNA signal comparable with that in seeds supplied with GA (Fig. 3A). Chi9 mRNA accumulation was detected after 1 d of imbibition of gib-1 seeds in 100 μm MeJA, and the maximum signal occurred after 2 d of incubation (Fig. 3A). However, unlike GA, MeJA did not stimulate germination of the gib-1 seeds. Chi9 protein was detected after 2 d of incubation in GA or MeJA, 1 d after the appearance of its mRNA signal (Fig. 3B). Low amounts of Chi9 mRNA detected in the absence of GA in northern blots (Fig. 3A) and in some tissue prints (Fig. 2) resulted in only faint or undetectable Chi9 protein bands (Fig. 3B). Chitinase activity assays from these protein extracts are consistent with the results of the immunoblot (Fig. 3B). GluB protein, on the other hand, did not accumulate in response to MeJA treatment (Fig. 3B). Chi9 transcript was induced by MeJA in a dose-dependent manner, with a 4-fold increase at 1 μm MeJA and a 15-fold increase at 100 μm MeJA relative to water-treated seeds (Fig. 3C).
Figure 3.
Induction of tomato Chi9 in gib-1 tomato seeds after GA or MeJA treatment. A, Gel-blot assay of Chi9 mRNA from gib-1 tomato seeds imbibed in water, in 100 μm GA4+7 (GA), in 0.05% (v/v) Tween 20 (Tween), or in 100 μm MeJA in 0.05% (v/v) Tween 20 solution at 25°C after 1, 2, or 3 d of imbibition. The bottom panel shows rRNA to verify equal loading (20 μg) of total RNA in each lane. B, Immunoblot analyses of Chi9 and GluB protein accumulation in gib-1 tomato seeds in response to 100 μm GA4+7 or MeJA. Seeds were imbibed as in A and tissues from the micropylar region of the seeds (endosperm cap and enclosed radicle tip) were dissected at the times indicated and proteins were extracted. Proteins from 48-h water-imbibed wild-type cv MM seeds were used as a positive control (left lane). Proteins were detected using antiserum against tobacco class I chitinase (top) and β-1,3-glucanase (bottom). Nonspecific cross-hybridization to another protein in the bottom panel indicates that equal amounts of protein (50 μg) were loaded in each lane. Chitinase activities in the same extracts (means ± se; n = 3) are shown below each lane. Means were compared first by analysis of variance, and if significant, mean differences were identified by Duncan's multiple range test. Means followed by the same letter are not significantly different (P < 0.05). C, Accumulation of Chi9 mRNA as a function of MeJA concentration. Total RNA was isolated from gib-1 tomato seeds after imbibition for 48 h in the indicated concentrations of MeJA in 0.05% (v/v) Tween 20 solution. Equal amounts (10 μg) of total RNA were loaded in each lane (bottom panel). The intensity of hybridization was measured with a Storm PhosphorImager (Molecular Dynamics, Sunnyvale, CA), normalized by rRNA amounts in each lane, and is shown relative to the signal in water-imbibed gib-1 seeds (lane 1 = 1 unit) below each lane.
Expression of Chi9 mRNA Is Reduced in Jasmonate-Deficient Seeds
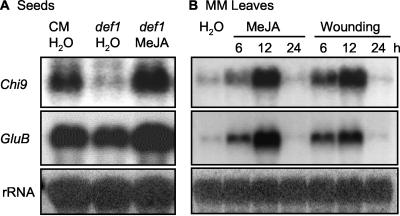
Jasmonate-deficient defenseless1 (def1) mutant tomato seeds (Howe et al., 1996) were used to test whether Chi9 mRNA accumulation is regulated in vivo by JA. In comparison with seeds of cv Castle-mart (CM), the wild-type background of def1, Chi9 mRNA accumulation was less abundant in def1 seeds after 24 h of imbibition (Fig. 4A). When def1 seeds were imbibed in 100 μm MeJA, Chi9 expression was restored to the level found in wild-type cv CM seeds (Fig. 4A). In contrast, expression of GluB transcript was similar in cv CM and def1 seeds (Fig. 4A).
Figure 4.
Tomato Chi9 and GluB expression in germinating seeds of the jasmonate-deficient defenseless1 (def1) mutant and its wild-type parent cv CM (A) or in wild-type cv MM leaves (B). A, Total RNA was extracted from seeds imbibed in water or in 100 μm MeJA for 24 h. B, Total RNA was isolated from leaves after the indicated times from treatment with water, 100 μm MeJA, or mechanical wounding. Equal amounts (10 μg) of RNA were loaded in each lane for hybridization analysis using Chi9 or GluB riboprobes.
Chi9 and GluB Show Tissue-Specific Regulation in Seeds and Leaves
To determine whether the induction of Chi9 by MeJA is unique to seeds, time courses of mRNA accumulations of Chi9 and GluB in MM tomato leaves were investigated after MeJA or wounding treatments. Expression of both genes was very low in the control sample, but after MeJA spraying or mechanical wounding, Chi9 and GluB mRNAs were detected at 6 h, increased at 12 h, then decreased back to initial levels by 24 h (Fig. 4B). Therefore, unlike in seeds, both of these genes were induced equally in tomato leaves in response to MeJA or mechanical wounding, and their expression kinetics were similar and transient.
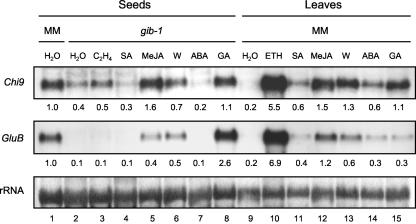
To further compare the regulation of these two genes in leaves versus seeds, we examined their expression in gib-1 seeds or cv MM leaves after treatment with ethylene, SA, MeJA, ABA, GA, or wounding. Chi9 and GluB were strongly induced in leaves by ethylene (27- and 34-fold, respectively) when compared with water-treated leaves (Fig. 5, lanes 9 and 10). However, ethylene had little or no effect on Chi9 and GluB expression in imbibed gib-1 seeds (Fig. 5, lanes 2 and 3). Although MeJA and wounding stimulated transcript accumulation of both genes in gib-1 seeds and cv MM leaves (Fig. 5, lanes 5, 6, 12, and 13), the induction of GluB mRNA expression was less than that of Chi9, consistent with detection of little GluB mRNA and protein accumulation in seeds in response to these factors (Figs. 1, A and C, 2, and 3B). On the other hand, GluB was strongly induced by GA treatment in gib-1 seeds but not in cv MM leaves, whereas GA induced Chi9 expression in both tissues (Fig. 5, lanes 8 and 15). SA and ABA were relatively ineffective in inducing Chi9 and GluB mRNA expression in either tissue (Fig. 5, lanes 4, 7, 11, and 14).
Figure 5.
Expression of tomato Chi9 and GluB mRNAs in response to different phytohormones or to wounding in imbibed wild-type cv MM or gib-1 tomato seeds or cv MM leaves. Seeds were imbibed for 48 h in water, 1 mm SA, 100 μm MeJA, 100 μm ABA, or 100 μm GA4+7 (GA) at 25°C before total RNA extraction. For ethylene (C2H4) treatment, gib-1 seeds were imbibed in water and were exposed to 10 μL L-1 ethylene for 48 h in a sealed glass chamber at 25°C. Twelve hours after spraying with phytohormone or wounding (W), tomato leaves were harvested from the first and second (above the cotyledon) fully expanded leaves of 3- to 4-week-old cv MM seedlings for total RNA isolation. Ethephon (ETH) aqueous solution (5 mm) was sprayed on the leaves for ethylene treatment. Total RNA (10 μg) was loaded in each well for RNA gel-blot analysis. Chi9 and GluB transcripts were quantified by a Storm PhosphorImager (Molecular Dynamics) and were normalized by the rRNA in each lane. The numbers below each lane in the top two panels give expression levels relative to water-imbibed cv MM seeds (lane 1 = 1 unit). Lane numbers are indicated below the bottom panel.
DISCUSSION
We investigated the hypothesis that Chi9 and GluB expression in germinating tomato seeds is related to wounding associated with tissue weakening and radicle protrusion through the endosperm cap. The Chi9 gene was induced in seeds to some extent by any wounding treatment tested (Fig. 1, A and B). Interestingly, when the lateral endosperm at the chalazal end of the seed was wounded, Chi9 mRNA accumulation was detected in the micropylar endosperm cap tissue, but not in the tissue adjacent to the wound site (Fig. 1, A and B). Similarly, nicking or piercing the chalazal end of the lateral endosperm induced Chi9 mRNA expression only in the micropylar tissues, even though radicle emergence did not occur (Fig. 1, A and B). This is surprising, for although there are transmissible wounding signals, most wounding responses are also expressed in the wounded tissue or in the cells adjacent to the damaged cells (e.g. Lee and Howe, 2003). For example, peroxidase activity was detected at the wound site in tomato lateral endosperm after cutting (Morohashi, 2002). In all cases, GluB mRNA was below detection in tissue prints of wounded gib-1 seeds (Fig. 1A), and little or no GluB protein was present (Fig. 1C), although a small increase in mRNA in response to wounding was detected by gel-blot hybridization (Fig. 5). Thus, wounding can induce expression of Chi9, and of GluB to a limited extent, but the response is exhibited only in the micropylar tissue regardless of where the seed is wounded.
Our results are in contrast with those of Morohashi and Matsushima (2000), who reported that wounding (cutting or puncturing) imbibed cv First Up tomato seeds enhanced β-1,3-glucanase activity in the lateral endosperm 1 d later. Chitinase activity, on the other hand, did not respond to the wounding treatments. Our results cannot eliminate the possibility that wounding can induce other tomato β-1,3-glucanase or chitinase genes because we used only GluB and Chi9 riboprobes at high stringency in our tissue prints and RNA blots. However, we previously showed that Chi9 and GluB were the only tomato chitinase and β-1,3-glucanase genes normally expressed during germination (Wu et al., 2001). The enzyme activity assayed by Morohashi and Matsushima (2000) might be due to wound induction of other tomato β-1,3-glucanase genes, which would not be detected by northern tissue prints, although, if present, they would likely have been detected by immunoblots with polyclonal antiserum that detects all classes of β-1,3-glucanases (Fig. 1C; Leubner-Metzger et al., 1998). It is also possible that tomato varieties differ in their gene expression patterns during germination and in response to wounding.
Because a wound signal apparently traveled from the wounded tissue to the endosperm cap, we tested whether second messengers known to be involved in wound signaling were involved. Ethylene, which can induce expression of many pathogenesis- and wound-related genes (Zhou, 1999; Leon et al., 2001) and was a potent inducer of GluB and Chi9 genes in leaves (Fig. 5), hardly affected expression of either gene in gib-1 tomato seeds (Figs. 2 and 5). Ethylene-dependent and ethylene-independent regulation of β-1,3-glucanases and chitinases was reported in pea (Pisum sativum) seeds, depending upon the stage of development (Petruzzelli et al., 1999). It is also possible that ethylene action is dependent upon the presence of GA, as in tobacco seeds (Leubner-Metzger et al., 1998), preventing an effect of ethylene in gib-1 seeds. However, the normal expression of both genes in nr tomato seeds (Fig. 2) further indicated that ethylene action is not required for the induction of Chi9 and GluB genes during germination. SA was ineffective in inducing expression of GluB and Chi9 in gib-1 tomato seeds or leaves (Figs. 2 and 5). Although SA induced β-1,3-glucanase and chitinase in several plants (Zhou, 1999), others have had results similar to ours (Christ and Mosinger, 1989; van Kan et al., 1995). ABA has been proposed to be a component in the signal transduction pathway of wounding responses leading to defense gene activation in tomato leaves (Peña-Cortés et al., 1989; Peña-Cortés and Willmitzer, 1995), although this has been questioned by others (Birkenmeier and Ryan, 1998). In our case, Chi9 and GluB expression was not induced by ABA in gib-1 tomato seeds and only a slight response was detected in cv MM leaves (Fig. 5). Tissue printing analyses of sitw tomato seeds (Fig. 2) also demonstrated that ABA is not required for induction of Chi9 and GluB genes during germination.
In contrast, MeJA was highly effective in inducing Chi9 mRNA, protein expression, and enzyme activity (Figs. 2, 3, 4, 5). As occurs during germination and after wounding (Figs. 1, A and B, and 2), Chi9 mRNA was found only in the endosperm cap tissue in response to MeJA (Fig. 2). To obtain more conclusive evidence that Chi9 expression is regulated by JA in vivo, we investigated Chi9 and GluB gene expression in def1 tomato seeds, a mutant in which the conversion of hydroperoxylinolenic acid to 12-oxo-phytodienoic acid in the JA biosynthetic pathway is blocked (Howe et al., 1996). Accumulation of wound-induced JA in the leaves of def1 tomato is reduced to around 30% of wild-type levels, resulting in less activation of defense genes after mechanical injury (Howe et al., 1996). Consistent with the regulation of Chi9 by MeJA, expression of Chi9 mRNA was much reduced during germination of def1 seeds after 24 h imbibition and was restored to wild-type levels by MeJA (Fig. 4A). Because def1 seeds complete germination and express GluB mRNA (Fig. 4A), they apparently are not limited for GA content or sensitivity, suggesting that JA acts downstream of GA in regulating Chi9 expression and that JA is not required for induction of GluB during germination. Taken together, the results indicate that JA may be a primary regulator of Chi9 expression during tomato seed germination. Indirect evidence supporting a role for JA during germination comes from recent proteomic studies of Arabidopsis seeds in which two JA-inducible proteins were identified that increased strongly at radicle emergence (Gallardo et al., 2001, 2002). In addition, JA-insensitive mutants of Arabidopsis exhibit increased sensitivity to inhibition of germination by ABA (Staswick et al., 1992; Berger et al., 1996; Ellis and Turner, 2002).
Although the tissue-specific expression of GluB and Chi9 normally occurs simultaneously in germinating tomato seeds (Wu et al., 2001), the two genes are regulated differently. For example, although both genes were induced in gib-1 seeds in response to GA, ABA suppressed expression of GluB but not of Chi9 in cv MM seeds (Wu et al., 2001). In the present study, Chi9 was expressed in seeds in response to wounding (Figs. 1 and 5) or MeJA (Figs. 2 and 3), whereas GluB was not (Fig. 1) or was only weakly responsive (Fig. 5). Particularly intriguing is the discovery that Chi9, but not GluB, was induced specifically in the micropylar tissues even when wounding occurred in other parts of the seed. Thus, not only does JA regulate Chi9 expression in tomato seeds, but there also appears to be a transmissible signal that can induce Chi9 expression specifically in the micropylar tissue. Whether this signal is JA or other components of wound signal transmission (e.g. systemin; Lee and Howe, 2003) and how the signal is transmitted to the micropylar tissue remains to be determined.
Furthermore, Chi9 and GluB exhibited distinct responses, particularly to ethylene and GA, in seed versus leaf tissues (Fig. 5). Although Chi9 and GluB expression in tomato seeds requires GA, this may be an indirect response to GA in the case of Chi9. The reduced expression of Chi9 in def1 seeds and its induction by MeJA in def1 and gib-1 seeds suggest that JA acts downstream of or independently from GA. Thus, the induction of Chi9 expression by GA in gib-1 seeds could be a consequence of the stimulation of germination, resulting in endosperm cell wall degradation and generation of a subsequent JA signal. On the other hand, expression of Chi9 was responsive to GA in cv MM leaves (Fig. 5), where overt wounding was not involved, so the responsiveness to GA or the requirement for JA apparently varies among tissues. JA does not appear to be required in the signaling pathway for GluB in seeds, as expression was unaffected in the def1 mutant (Fig. 4A). In further contrast to Chi9, GA stimulated GluB expression in seeds but not in leaves (Fig. 5), indicating that the GluB promoter is responsive to GA in a tissue-specific manner, or that a second messenger other than JA (or other elicitors tested here) is involved in the induction of GluB in seeds in response to GA. As for tobacco class I β-1,3-glucanase and chitinase genes (Leubner-Metzger et al., 1998; Rezzonico et al., 1998), the distinct regulatory patterns of Chi9 and GluB in tomato are likely due to different hormone-responsive elements in the promoter regions of these two genes, the activities of which are further modified by the tissue type.
Although Chi9 and GluB are normally expressed together in the endosperm cap tissue during tomato seed germination, we found no evidence to support a functional role for these enzymes in weakening of this tissue (Wu et al., 2001), although they could perform other developmental functions (Gomez et al., 2002; Leubner-Metzger, 2003). On the other hand, it is well known that chitinase and β-1,3-glucanase are components of a broad-spectrum plant defense mechanism (van Loon, 1999), and many authors have proposed that chitinase and/or β-1,3-glucanase expression in developing and germinating seeds may represent a prophylactic or inducible mechanism for protection against microbial invasion (Fincher, 1989; Leah et al., 1991; Cordero et al., 1994; Høj and Fincher, 1995; Caruso et al., 1999; Petruzzelli et al., 1999; Morohashi and Matsushima, 2000; Wu et al., 2001; Gomez et al., 2002; Whitmer et al., 2003). During seed germination, radicle emergence will expose the inner tissues of the seed and create an avenue for entry of microorganisms into the storage reserves. In tomato, Chi9 and GluB are induced specifically in the micropylar endosperm, which forms a collar around the emerged radicle, just before radicle protrusion (Wu et al., 2001). Instead of mounting a general defense throughout the whole endosperm tissue, the space- and time-specific expression pattern of Chi9 and GluB would concentrate such a defensive mechanism in the “frontline” tissues of the micropylar endosperm to act directly by degrading cell walls of invading fungi or indirectly by releasing oligosaccharide elicitors of defense responses. The vacuolar targeting of these enzymes is consistent with the model proposed by Mauch and Staehelin (1989) in which hyphae that penetrate a cell would be exposed to a potentially lethal concentration of β-1,3-glucanase and chitinase.
We hypothesize that the induction of Chi9 during tomato seed germination is associated with a “wounding-like” response from cell wall hydrolysis and weakening of the endosperm cap and that JA is involved in the signaling cascade for this response downstream from or parallel to GA action. GluB, on the other hand, although expressed at the same time as Chi9 during germination, is regulated primarily by GA and ABA rather than JA. Together, these enzymes may function to delay the entry of pathogenic or saprophytic microorganisms into the endosperm. Because Chi9 and GluB are not expressed at wound sites on the lateral endosperm, this hypothesis can be tested by determining the success of microbial penetration through such sites compared with that in seeds germinating through the endosperm cap where these enzymes are present. The mutants and hormonal treatments described here would allow manipulation of whether both enzymes are present simultaneously or individually to further examine this hypothesis.
MATERIALS AND METHODS
Plant Material and Seed Germination
The tomato (Lycopersicon esculentum) seeds used were harvested from field-grown plants in Davis, CA, except that seeds of sitw, def1, and its wild-type parent cv CM were produced in a greenhouse. The gib-1 mutant plants were sprayed three times per week with 100 μm GA4+7 to revert the dwarf habit and to allow more vigorous growth and fertility. The sitw mutant plants were sprayed once per week with 100 μm ABA to reduce wilting and stimulate growth. After extraction and drying, the seeds were stored at -20°C until used. Tomato seeds were sterilized in 10% (v/v) bleach (0.5% [w/v] sodium hypochlorite) and 1% (v/v) Tween 20 solution on a shaker for 10 min and were then rinsed six times with distilled water. The seeds were sown on two layers of germination blotters in 9-cm-diameter petri dishes moistened with 12 mL of deionized water or the chemical solution indicated and were incubated at 25°C in the dark (Ni and Bradford, 1993). For tomato leaf samples used in this study, wild-type cv MM tomato plants were grown in a growth chamber with fluorescent lights at 700 μE m-2 s-1 and cycling temperatures of 25°C (light, 17 h) and 20°C (dark, 7 h).
Wounding and Elicitor Treatments
For wounding treatments, sterilized tomato seeds were first imbibed on autoclaved blotter paper moistened with sterile deionized water for 12 h in the germination conditions described above. Each seed was then wounded on the endosperm of its chalazal end by cutting off a piece (cut treatment), nicking with a blade without removing tissue (nicking treatment), or piercing with a pin (piercing treatment). The seeds were further incubated in the same conditions for the times indicated.
Ethylene treatment was performed in a 5-liter sealed glass chamber with ethylene concentrations as indicated at 5.5 L h-1 flow rate at 25°C. The concentration of ethylene gas was monitored every 12 h by gas chromatography (Series 100; Carle AGC, Loveland, CO, equipped with alumina column and a flame ionization detector).
Sodium SA, FC, ABA, ethephon (2-chloroethylphosphonic acid), and BABA were obtained from Sigma (St. Louis). MeJA was purchased from Aldrich (Milwaukee, WI). GA4+7 was provided by Abbott Laboratories (North Chicago). Treatment solutions were diluted from 1 mm stock solutions prepared in water. MeJA was first dissolved in a small amount ethanol, and was then brought to final volume in 0.05% (v/v) Tween 20.
Tomato leaf samples were harvested from the first and second fully expanded leaves (above the cotyledons) of six 3- to 4-week-old seedlings after spraying with elicitor chemical in 0.05% (v/v) Tween 20 solution or with Tween 20 solution only as a control. For mechanical injury treatment, a single wound was inflicted perpendicular to the midvein near the tip of the leaflet using a hemostat (Howe et al., 1996). Samples were frozen immediately in liquid nitrogen and were stored at -80°C until used.
Chitinase Activity Assay
Chitinase activity was assayed colorimetrically using Remazol Brilliant Violet-labeled carboxymethyl-chitin (CM-chitin-RBV; 2 mg mL-1; Loewe Biochemica, Mühlweg, Germany) as the substrate using a method modified from Wirth and Wolf (1992). Each assay was performed in a 0.5-mL microfuge tube containing 50 μL of CM-chitin-RBV, 100 μL 100 mm sodium acetate buffer (pH 5.0), and 50 μL of tomato seed extract or standard (purified chitinase from Serratia marcescens; Sigma). After incubating in a 37°C water bath for 2.5 h (Pegg, 1988), the enzyme reaction was terminated by cooling the tube on ice for 10 min before 50 μL of 0.3 n HCl was added to precipitate nondegraded CM-chitin-RBV. After centrifugation (1,450g for 10 min at 4°C), the supernatant (200 μL) was transferred to a microcuvette and A550 was measured spectrophotometrically (UV-160U; Shimadzu, Columbia, MD) against a blank (incubation mixture without enzyme or sample added).
Analysis of Proteins, RNAs, and Tissue Printing
Protein extraction and immunoblot analysis, RNA extraction and gel-blot analysis, and tissue printing were conducted as described previously (Wu et al., 2001).
Acknowledgments
We thank Dr. Clarence A. Ryan (Washington State University, Pullman, WA) for kindly providing def1 and cv CM tomato stock seeds. Antibodies against tobacco class I chitinase and β-1,3-glucanase were the generous gift of Dr. Frederick Meins, Jr. (Friedrich Miescher-Institut, Basel, Switzerland).
This work was supported in part by the National Science Foundation (grant no. IBN-9722978) and by the National Research Initiative Competitive Grants Program of the U.S. Department of Agriculture (grant no. 2001-35304).
References
- Anfoka G, Buchenauer H (1997) Systemic acquired resistance in tomato against Phytophthora infestans by pre-inoculation with tobacco necrosis virus. Physiol Mol Plant Pathol 50: 85-90 [Google Scholar]
- Beaudoin N, Serizet C, Gosti F, Giraudat J (2000) Interactions between abscisic acid and ethylene signaling cascades. Plant Cell 12: 1103-1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S, Bell E, Mullet JE (1996) Two methyl jasmonate-insensitive mutants show altered expression of AtVSP in response to methyl jasmonate and wounding. Plant Physiol 111: 525-531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD (1997) Breaking down the walls: a role for endo-β-mannanase in release from seed dormancy? Trends Plant Sci 2: 464-469 [Google Scholar]
- Birkenmeier GF, Ryan CA (1998) Wound signaling in tomato plants: evidence that ABA is not a primary signal for defense gene activation. Plant Physiol 117: 687-693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso C, Chilosi G, Caporale C, Leonardi L, Bertini L, Magro P, Buonocore V (1999) Induction of pathogenesis-related proteins in germinating wheat seeds infected with Fusarium culmorum. Plant Sci 140: 87-97 [Google Scholar]
- Chao WS, Gu Y-Q, Pautot V, Bray EA, Walling LL (1999) Leucine aminopeptidase RNAs, proteins, and activities increase in response to water deficit, salinity, and the wound signals systemin, methyl jasmonate, and abscisic acid. Plant Physiol 120: 979-992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Bradford KJ (2000) Expression of an expansin is associated with endosperm weakening during tomato seed germination. Plant Physiol 124: 1265-1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Nonogaki H, Bradford KJ (2002) A gibberellin-regulated xyloglucan endotransglycosylase gene is expressed in the endosperm cap during tomato seed germination. J Exp Bot 53: 215-223 [DOI] [PubMed] [Google Scholar]
- Christ U, Mosinger E (1989) Pathogenesis-related proteins of tomato: induction by Phytophthora infestans and other biotic and abiotic inducers and correlations with resistance. Physiol Mol Plant Pathol 35: 53-65 [Google Scholar]
- Cohen Y, Niderman T, Mosinger E, Fluhr R (1994) β-Aminobutyric acid induces the accumulation of pathogenesis-related proteins in tomato (Lycopersicon esculentum) plants and resistance to late blight infection caused by Phytophthora infestans. Plant Physiol 104: 59-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero MJ, Raventos D, San Segundo B (1994) Differential expression and induction of chitinases and β-1, 3-glucanases in response to fungal infection during germination of maize seeds. Mol Plant-Microbe Interact 7: 23-31 [Google Scholar]
- Danhash N, Wagemakers CAM, van Kan JAL, de Wit PJGM (1993) Molecular characterization of four chitinase cDNAs obtained from Cladosporium fulvum-infected tomato. Plant Mol Biol 22: 1017-1029 [DOI] [PubMed] [Google Scholar]
- Ellis C, Turner JG (2002) A conditionally fertile coi1 allele indicates crosstalk between plant hormone signalling pathways in Arabidopsis thaliana seeds and young seedlings. Planta 215: 549-556 [DOI] [PubMed] [Google Scholar]
- Feurtado JA, Banik M, Bewley JD (2001) The cloning and characterization of α-galactosidase present during and following germination of tomato (Lycopersicon esculentum Mill.) seed. J Exp Bot 52: 1239-1249 [PubMed] [Google Scholar]
- Fincher GB (1989) Molecular and cellular biology associated with endosperm mobilization in germinating cereal grains. Annu Rev Plant Physiol Plant Mol Biol 40: 305-346 [Google Scholar]
- Gallardo K, Job C, Groot SPC, Puype M, Demol H, Vandekerckhove J, Job D (2001) Proteomic analysis of Arabidopsis seed germination and priming. Plant Physiol 126: 835-848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo K, Job C, Groot SPC, Puype M, Demol H, Vandekerckhove J, Job D (2002) Proteomics of Arabidopsis seed germination. a comparative study of wild-type and gibberellin-deficient seeds. Plant Physiol 129: 823-837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P (2000) Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell 12: 1117-1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez L, Allona I, Casado R, Aragoncillo C (2002) Seed chitinases. Seed Sci Res 12: 217-230 [Google Scholar]
- Groot SPC, Karssen CM (1987) Gibberellins regulate seed germination in tomato by endosperm weakening: a study with gibberellin-deficient mutants. Planta 171: 525-531 [DOI] [PubMed] [Google Scholar]
- Groot SPC, Karssen CM (1992) Dormancy and germination of abscisic acid-deficient tomato seeds: studies with the sitiens mutant. Plant Physiol 99: 952-958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høj PB, Fincher GB (1995) Molecular evolution of plant β-glucan endohydrolases. Plant J 7: 367-379 [DOI] [PubMed] [Google Scholar]
- Howe GA, Lightner J, Browse J, Ryan CA (1996) An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. Plant Cell 8: 2067-2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongedijk E, Tigelaar H, van Rekel JSC, BrespVloemans SA (1995) Synergistic activity of chitinases and β-1,3-glucanases enhances fungal resistance in transgenic tomato plants. Euphytica 85: 173-180 [Google Scholar]
- Klee H, Tieman D (2002) The tomato ethylene receptor gene family: form and function. Physiol Plant 115: 336-341 [DOI] [PubMed] [Google Scholar]
- Lawrence CB, Josten MHAJ, Tuzun S (1996) Differential induction of pathogenesis-related proteins in tomato by Alternaria solani and the association of basic chitinase isozyme with resistance. Physiol Mol Plant Pathol 48: 361-377 [Google Scholar]
- Leah R, Tommerup H, Svendsen I, Mundy J (1991) Biochemical and molecular characterization of three barley seed proteins with antifungal properties. J Biol Chem 266: 1564-1573 [PubMed] [Google Scholar]
- Lee GI, Howe GA (2003) The tomato mutant spr1 is defective in systemin perception and the production of a systemic wound signal for defense gene expression. Plant J 33: 567-576 [DOI] [PubMed] [Google Scholar]
- Leon J, Rojo E, Sanchez-Serrano JJ (2001) Wound signalling in plants. J Exp Bot 52: 1-9 [DOI] [PubMed] [Google Scholar]
- Leubner-Metzger G (2003) Functions and regulation of β-1,3-glucanases during seed germination, dormancy release and after-ripening. Seed Sci Res 13: 17-34 [Google Scholar]
- Leubner-Metzger G, Fründt C, Meins FJ (1996) Effects of gibberellins, darkness and osmotica on endosperm rupture and class I β-1, 3-glucanase induction in tobacco seed germination. Planta 199: 282-288 [Google Scholar]
- Leubner-Metzger G, Fründt C, Vögeli-Lange R, Meins FJ (1995) Class I β-1, 3-glucanases in the endosperm of tobacco during germination. Plant Physiol 109: 751-759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leubner-Metzger G, Meins FJ (1999) Function and regulation of plant β-1, 3-glucanases. In SK Datta, S Muthukrishnan, eds, Pathogenesis-Related Proteins in Plants. CRC Press, Boca Raton, FL, pp 49-76
- Leubner-Metzger G, Meins F (2000) Sense transformation reveals a novel role for class I β-1,3-glucanase in tobacco seed germination. Plant J 23: 215-221 [DOI] [PubMed] [Google Scholar]
- Leubner-Metzger G, Petruzzelli L, Waldvogel R, Vögeli-Lange R, Meins F Jr (1998) Ethylene-responsive element binding protein (EREBP) expression and the transcriptional regulation of class I β-1,3-glucanase during tobacco seed germination. Plant Mol Biol 38: 785-795 [DOI] [PubMed] [Google Scholar]
- Li L, Li C, Lee GI, Howe GA (2002) Distinct roles for jasmonate synthesis and action in the systemic wound response of tomato. Proc Natl Acad Sci USA 99: 6416-6421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone M, Alarcon JJ (1995) Only xylem-borne factors can account for systemic wound signaling in the tomato plant. Planta 196: 740-746 [Google Scholar]
- Mauch F, Staehelin LA (1989) Functional implications of the subcellular localization of ethylene-induced chitinase and β-1,3-glucanase in bean leaves. Plant Cell 1: 447-457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConn M, Creelman RA, Bell E, Mullet JE, Browse J (1997) Jasmonate is essential for insect defense in Arabidopsis. Proc Natl Acad Sci USA 94: 5473-5477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meins FJ, Neuhaus J-M, Sperisen C, Ryals J (1992) The primary structure of plant pathogenesis-related glucanohydrolases and their genes. In T Boller, F Meins, eds, Genes Involved in Plant Defense. Springer-Verlag, Berlin, pp 245-282
- Mo B, Bewley JD (2002) α-Mannosidase (EC 3.2.1.25) activity during and following germination of tomato (Lycopersicon esculentum Mill.) seeds: purification, cloning and characterization. Planta 215: 141-152 [DOI] [PubMed] [Google Scholar]
- Morohashi Y (2002) Peroxidase activity develops in the micropylar endosperm of tomato seeds prior to radicle protrusion. J Exp Bot 53: 1643-1650 [DOI] [PubMed] [Google Scholar]
- Morohashi Y, Matsushima H (2000) Development of β-1,3-glucanase activity in germinated tomato seeds. J Exp Bot 51: 1381-1387 [PubMed] [Google Scholar]
- Neuhaus J-M (1999) Plant chitinases (PR-3, PR-4, PR-8, PR-11). In SK Datta, S Muthukrishnan, eds, Pathogenesis-Related Proteins in Plants. CRC Press, Boca Raton, FL, pp 77-105
- Ni BR, Bradford KJ (1992) Quantitative models characterizing seed germination responses to abscisic acid and osmoticum. Plant Physiol 98: 1057-1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni B-R, Bradford KJ (1993) Germination and dormancy of abscisic acid- and gibberellin-deficient mutant tomato (Lycopersicon esculentum) seeds; sensitivity of germination to abscisic acid, gibberellin, and water potential. Plant Physiol 101: 607-617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonogaki H, Gee OH, Bradford KJ (2000) A germination-specific endo-β-mannanase gene is expressed in the micropylar endosperm cap of tomato seeds. Plant Physiol 123: 1235-1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg GF (1988) Chitinase from tomato Lycopersicon esculentum. Methods Enzymol 161: 484-489 [Google Scholar]
- Peña-Cortés H, Sanchez-Serrano JJ, Mertens R, Willmitzer L, Prat S (1989) Abscisic acid is involved in the wound-induced expression of the proteinase inhibitor II gene in potato and tomato. Proc Natl Acad Sci USA 86: 9851-9855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña-Cortés H, Willmitzer L (1995) The role of hormones in gene activation in response to wounding. In PJ Davies, ed, Plant Hormones. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 395-414
- Petruzzelli L, Kunz C, Waldvogel R, Meins F Jr, Leubner-Metzger G (1999) Distinct ethylene- and tissue-specific regulation of β-1,3-glucanases and chitinases during pea seed germination. Planta 209: 195-201 [DOI] [PubMed] [Google Scholar]
- Rezzonico E, Flury N, Meins FJ, Beffa R (1998) Transcriptional down-regulation by abscisic acid of pathogenesis-related β-1,3-glucanase genes in tobacco cell cultures. Plant Physiol 117: 585-592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MR, Bowles DJ (1999) Fusicoccin, 14-3-3 proteins, and defense responses in tomato plants. Plant Physiol 119: 1243-1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller A, Roy P, Amrhein N (2000) Salicylic acid-independent induction of pathogenesis-related gene expression by fusicoccin. Planta 210: 599-606 [DOI] [PubMed] [Google Scholar]
- Schwartz SH, Qin X, Zeevaart AD (2003) Elucidation of the indirect pathway of abscisic acid biosynthesis by mutants, genes, and enzymes. Plant Physiol 131: 1591-1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela-Buurlage MB, Ponstein AS, Bres-Vloemans SA, Melchers LS, van den Elzen PJM, Cornelissen BJC (1993) Only specific tobacco (Nicotiana tabacum) chitinase and β-1,3-glucanase exhibit antifungal activity. Plant Physiol 101: 857-863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya N, Minami E (2001) Oligosaccharide signalling for defence responses in plant. Physiol Mol Plant Pathol 59: 223-233 [Google Scholar]
- Sitrit Y, Hadfield KA, Bennett AB, Bradford KJ, Downie B (1999) Expression of a polygalacturonase associated with tomato seed germination. Plant Physiol 121: 419-428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Su W, Howell SH (1992) Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc Natl Acad Sci USA 89: 6837-6840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stintzi A, Heitz T, Prasad V, Wiedemann-Merdinoglu S, Kauffmann S, Geoffroy P, Legrand M, Fritig B (1993) Plant “pathogenesis-related” proteins and their role in defense against pathogens. Biochimie 75: 687-706 [DOI] [PubMed] [Google Scholar]
- van Kan JAL, Cozijnsen T, Danhash N, De Wit PJG (1995) Induction of tomato stress protein mRNAs by ethephon, 2, 6-dichloroisonicotinic acid and salicylate. Plant Mol Biol 27: 1205-1213 [DOI] [PubMed] [Google Scholar]
- van Kan JAL, Joosten HAJ, Wagemakers CAM, van den Berg-Velthuis GCM, de Wit PJGM (1992) Differential accumulation of mRNAs encoding extracellular and intracellular PR proteins in tomato induced by virulent and avirulent races of Cladosporium fulvum. Plant Mol Biol 20: 513-527 [DOI] [PubMed] [Google Scholar]
- van Loon LC (1997) Induced resistance in plants and the role of pathogenesis-related proteins. Eur J Plant Pathol 103: 753-765 [Google Scholar]
- van Loon LC (1999) Occurrence and properties of plant pathogenesis-related proteins. In SK Datta, S Muthukrishnan, eds, Pathogenesis-Related Proteins in Plants. CRC Press, Boca Raton, FL, pp 1-19
- Whitmer X, Nonogaki H, Beers EP, Bradford KJ, Welbaum GE (2003) Characterization of chitinase activity and gene expression in muskmelon seeds. Seed Sci Res 13: 167-178 [Google Scholar]
- Wirth SJ, Wolf GA (1992) Micro-plate colourimetric assay for endo-acting cellulase, xylanase, chitinase, 1,3-β-glucanase and amylase extracted from forest soil horizons. Soil Biol Biochem 24: 511-519 [Google Scholar]
- Wu C-T, Leubner-Metzger G, Meins F, Bradford KJ (2001) Class I β-1, 3-glucanase and chitinase are expressed in the micropylar endosperm of tomato seeds prior to radicle emergence. Plant Physiol 126: 1299-1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J-M (1999) Signal transduction and pathogen-induced PR gene expression. In SK Datta, S Muthukrishnan, eds, Pathogenesis-Related Proteins in Plants. CRC Press, Boca Raton, FL, pp 195-205