Abstract
The bacteriochlorophyll biosynthesis gene, bchM, from Rhodobacter capsulatus was previously believed to code for a polypeptide involved in formation of the cyclopentone ring of protochlorophyllide from Mg-protoporphyrin IX monomethyl ester. In this study, R. capsulatus bchM was expressed in Escherichia coli and the gene product was subsequently demonstrated by enzymatic analysis to catalyze methylation of Mg-protoporphyrin IX to form Mg-protoporphyrin IX monomethyl ester. Activity required the substrates Mg-protoporphyrin IX and S-adenosyl-L-methionine. 14C-labeled product was formed in incubations containing 14C-methyl-labeled S-adenosyl-L-methionine. On the basis of these and previous results, we also conclude that the bchH gene, which was previously reported to code for Mg-protoporphyrin IX methyltransferase, is most likely involved in the Mg chelation step.
Full text
PDF

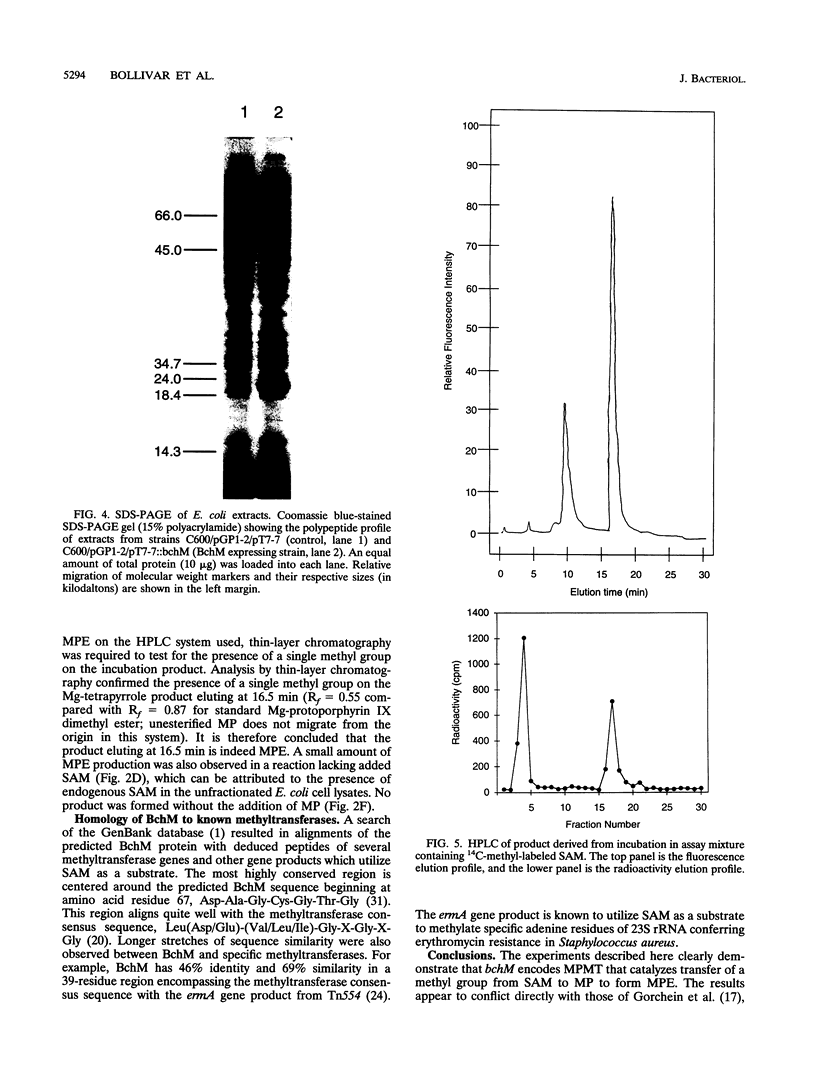


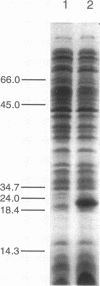
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Armstrong G. A., Alberti M., Leach F., Hearst J. E. Nucleotide sequence, organization, and nature of the protein products of the carotenoid biosynthesis gene cluster of Rhodobacter capsulatus. Mol Gen Genet. 1989 Apr;216(2-3):254–268. doi: 10.1007/BF00334364. [DOI] [PubMed] [Google Scholar]
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C. E., Bollivar D. W., Suzuki J. Y. Genetic analyses of photopigment biosynthesis in eubacteria: a guiding light for algae and plants. J Bacteriol. 1993 Jul;175(13):3919–3925. doi: 10.1128/jb.175.13.3919-3925.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Weiss A., Crossland L. Polarity of Tn5 insertion mutations in Escherichia coli. J Bacteriol. 1980 May;142(2):439–446. doi: 10.1128/jb.142.2.439-446.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biel A. J., Marrs B. L. Transcriptional regulation of several genes for bacteriochlorophyll biosynthesis in Rhodopseudomonas capsulata in response to oxygen. J Bacteriol. 1983 Nov;156(2):686–694. doi: 10.1128/jb.156.2.686-694.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollivar D. W., Bauer C. E. Nucleotide Sequence of S-Adenosyl-l-Methionine: Magnesium Protoporphyrin Methyltransferase from Rhodobacter capsulatus. Plant Physiol. 1992 Jan;98(1):408–410. doi: 10.1104/pp.98.1.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollivar D. W., Suzuki J. Y., Beatty J. T., Dobrowolski J. M., Bauer C. E. Directed mutational analysis of bacteriochlorophyll a biosynthesis in Rhodobacter capsulatus. J Mol Biol. 1994 Apr 15;237(5):622–640. doi: 10.1006/jmbi.1994.1260. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burke D. H., Alberti M., Hearst J. E. The Rhodobacter capsulatus chlorin reductase-encoding locus, bchA, consists of three genes, bchX, bchY, and bchZ. J Bacteriol. 1993 Apr;175(8):2407–2413. doi: 10.1128/jb.175.8.2407-2413.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D. H., Alberti M., Hearst J. E. bchFNBH bacteriochlorophyll synthesis genes of Rhodobacter capsulatus and identification of the third subunit of light-independent protochlorophyllide reductase in bacteria and plants. J Bacteriol. 1993 Apr;175(8):2414–2422. doi: 10.1128/jb.175.8.2414-2422.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debussche L., Couder M., Thibaut D., Cameron B., Crouzet J., Blanche F. Assay, purification, and characterization of cobaltochelatase, a unique complex enzyme catalyzing cobalt insertion in hydrogenobyrinic acid a,c-diamide during coenzyme B12 biosynthesis in Pseudomonas denitrificans. J Bacteriol. 1992 Nov;174(22):7445–7451. doi: 10.1128/jb.174.22.7445-7451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorchein A., Gibson L. C., Hunter C. N. Gene expression and control of enzymes for synthesis of magnesium protoporphyrin monomethyl ester in Rhodobacter sphaeroides. Biochem Soc Trans. 1993 May;21(2):201S–201S. doi: 10.1042/bst021201s. [DOI] [PubMed] [Google Scholar]
- Gorchein A. Magnesium protoporphyrin chelatase activity in Rhodopseudomonas spheroides. Studies with whole cells. Biochem J. 1972 Mar;127(1):97–106. doi: 10.1042/bj1270097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A., Carpenter R., Doyle S., Coen E. S. Olive: a key gene required for chlorophyll biosynthesis in Antirrhinum majus. EMBO J. 1993 Oct;12(10):3711–3719. doi: 10.1002/j.1460-2075.1993.tb06048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingrosso D., Fowler A. V., Bleibaum J., Clarke S. Sequence of the D-aspartyl/L-isoaspartyl protein methyltransferase from human erythrocytes. Common sequence motifs for protein, DNA, RNA, and small molecule S-adenosylmethionine-dependent methyltransferases. J Biol Chem. 1989 Nov 25;264(33):20131–20139. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marrs B. Mobilization of the genes for photosynthesis from Rhodopseudomonas capsulata by a promiscuous plasmid. J Bacteriol. 1981 Jun;146(3):1003–1012. doi: 10.1128/jb.146.3.1003-1012.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis K., Faloona F., Scharf S., Saiki R., Horn G., Erlich H. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):263–273. doi: 10.1101/sqb.1986.051.01.032. [DOI] [PubMed] [Google Scholar]
- Murphy E. Nucleotide sequence of ermA, a macrolide-lincosamide-streptogramin B determinant in Staphylococcus aureus. J Bacteriol. 1985 May;162(2):633–640. doi: 10.1128/jb.162.2.633-640.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsat B., Monfort A., Chatellard P., Stutz E. Mapping and sequencing of an actively transcribed Euglena gracilis chloroplast gene (ccsA) homologous to the Arabidopsis thaliana nuclear gene cs(ch-42). FEBS Lett. 1992 Jun 1;303(2-3):181–184. doi: 10.1016/0014-5793(92)80514-h. [DOI] [PubMed] [Google Scholar]
- Radmer R. J., Bogorad L. (Minus) S-adenosyl-L-methionine-magnesium protoporphyrin methyltransferase, an enzyme in the biosynthetic pathway of chlorophyll in Zea mays. Plant Physiol. 1967 Mar;42(3):463–465. doi: 10.1104/pp.42.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. P., Cohen S. N., Clark W. G., Marrs B. L. Alignment of genetic and restriction maps of the photosynthesis region of the Rhodopseudomonas capsulata chromosome by a conjugation-mediated marker rescue technique. J Bacteriol. 1983 May;154(2):580–590. doi: 10.1128/jb.154.2.580-590.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Arakawa E., Ito K., Kato J., Nakamura A. Genetic analysis of an invasion region by use of a Tn3-lac transposon and identification of a second positive regulator gene, invE, for cell invasion of Shigella sonnei: significant homology of invE with ParB of plasmid P1. J Bacteriol. 1990 Feb;172(2):619–629. doi: 10.1128/jb.172.2.619-629.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Y. S., Castelfranco P. A., Goff D. A., Smith K. M. Intermediates in the formation of the chlorophyll isocyclic ring. Plant Physiol. 1985 Nov;79(3):725–729. doi: 10.1104/pp.79.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsebo K. M., Hearst J. E. Genetic-physical mapping of a photosynthetic gene cluster from R. capsulata. Cell. 1984 Jul;37(3):937–947. doi: 10.1016/0092-8674(84)90428-8. [DOI] [PubMed] [Google Scholar]