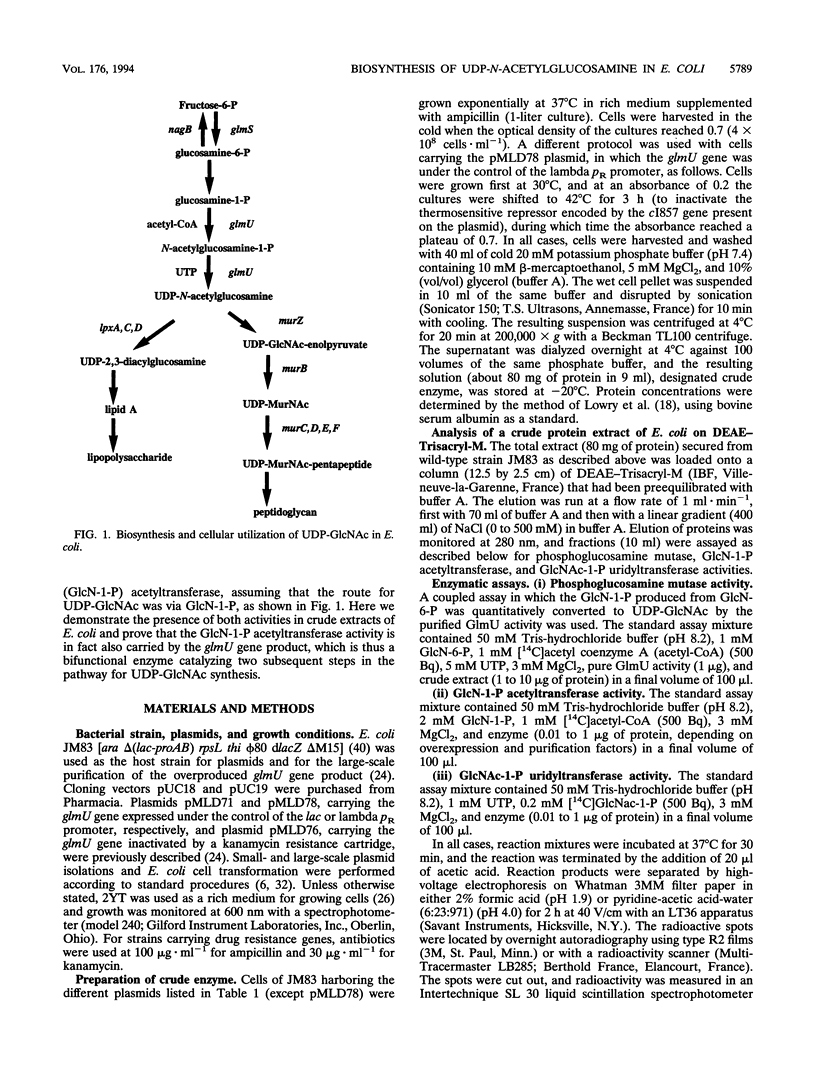
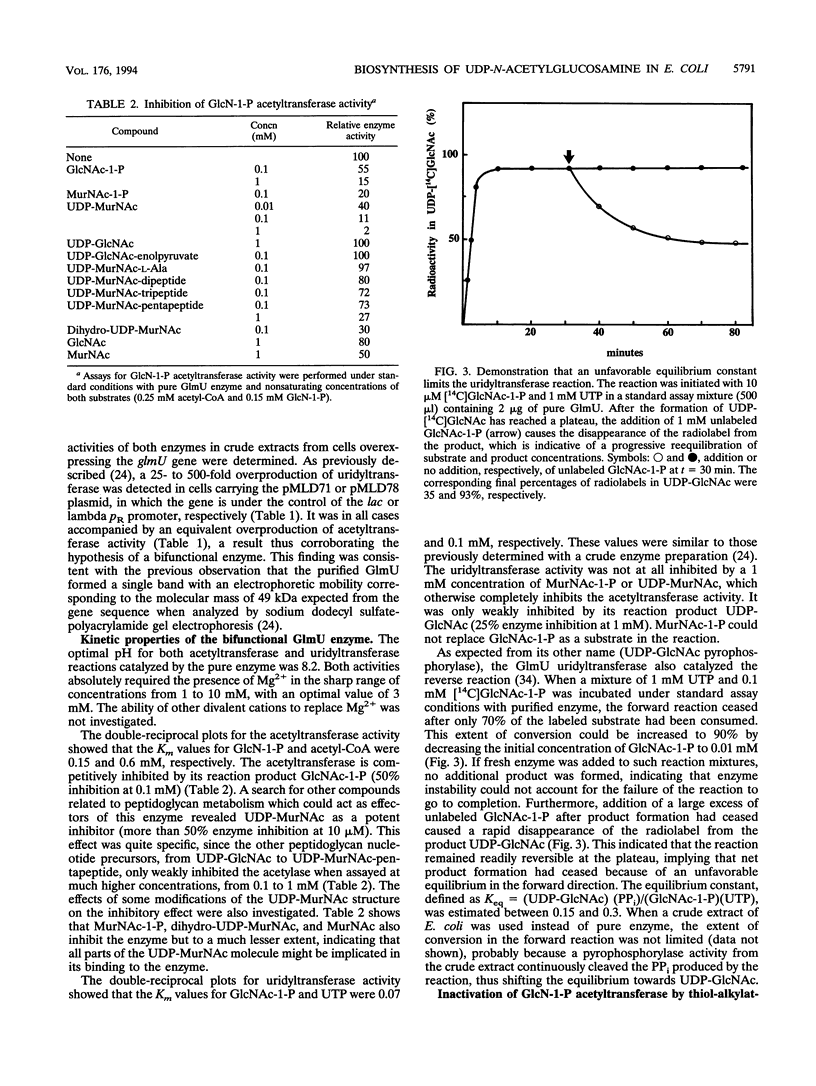
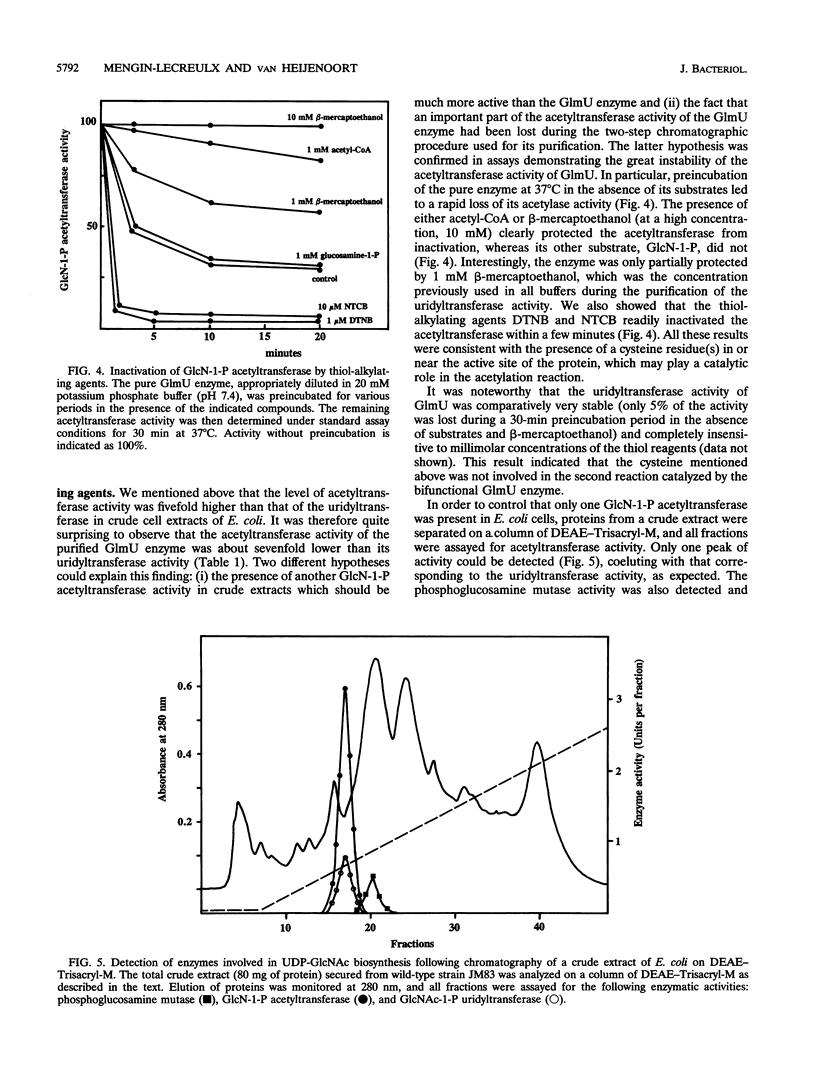
Abstract
The glmU gene product of Escherichia coli was recently identified as the N-acetylglucosamine-1-phosphate uridyltransferase activity which catalyzes the formation of UDP-N-acetylglucosamine, an essential precursor for cell wall peptidoglycan and lipopolysaccharide biosyntheses (D. Mengin-Lecreulx and J. van Heijenoort, J. Bacteriol. 175:6150-6157, 1993). Evidence that the purified GlmU protein is in fact a bifunctional enzyme which also catalyzes acetylation of glucosamine-1-phosphate, the preceding step in the same pathway, is now provided. Kinetic parameters of both reactions were investigated, indicating in particular that the acetyltransferase activity of the enzyme is fivefold higher than its uridyltransferase activity. In contrast to the uridyltransferase activity, which is quite stable and insensitive to thiol reagents, the acetyltransferase activity was rapidly lost when the enzyme was stored in the absence of reducing thiols or acetyl coenzyme A or was treated with thiol-alkylating agents, suggesting the presence of at least one essential cysteine residue in or near the active site. The acetyltransferase activity is greatly inhibited by its reaction product N-acetylglucosamine-1-phosphate and, interestingly, also by UDP-N-acetylmuramic acid, which is one of the first precursors specific for the peptidoglycan pathway. The detection in crude cell extracts of a phosphoglucosamine mutase activity finally confirms that the route from glucosamine-6-phosphate to UDP-N-acetylglucosamine occurs via glucosamine-1-phosphate in bacteria.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. S., Bull H. G., Galloway S. M., Kelly T. M., Mohan S., Radika K., Raetz C. R. UDP-N-acetylglucosamine acyltransferase of Escherichia coli. The first step of endotoxin biosynthesis is thermodynamically unfavorable. J Biol Chem. 1993 Sep 15;268(26):19858–19865. [PubMed] [Google Scholar]
- Anderson R. G., Douglas L. J., Hussey H., Baddiley J. The control of synthesis of bacterial cell walls. Interaction in the synthesis of nucleotide precursors. Biochem J. 1973 Dec;136(4):871–876. doi: 10.1042/bj1360871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R., Ames B. N. Complete analysis of cellular nucleotides by two-dimensional thin layer chromatography. J Biol Chem. 1982 Aug 25;257(16):9759–9769. [PubMed] [Google Scholar]
- Copeland J. C., Marmur J. Identification of conserved genetic functions in Bacillus by use of temperature-sensitive mutants. Bacteriol Rev. 1968 Dec;32(4 Pt 1):302–312. [PMC free article] [PubMed] [Google Scholar]
- Dobrogosz W. J. Effect of amino sugars on catabolite repression in Escherichia coli. J Bacteriol. 1968 Feb;95(2):578–584. doi: 10.1128/jb.95.2.578-584.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie J. A. The nodL gene from Rhizobium leguminosarum is homologous to the acetyl transferases encoded by lacA and cysE. Mol Microbiol. 1989 Nov;3(11):1649–1651. doi: 10.1111/j.1365-2958.1989.tb00150.x. [DOI] [PubMed] [Google Scholar]
- Dutka-Malen S., Mazodier P., Badet B. Molecular cloning and overexpression of the glucosamine synthetase gene from Escherichia coli. Biochimie. 1988 Feb;70(2):287–290. doi: 10.1016/0300-9084(88)90073-9. [DOI] [PubMed] [Google Scholar]
- Flouret B., Mengin-Lecreulx D., van Heijenoort J. Reverse-phase high-pressure liquid chromatography of uridine diphosphate N-acetylmuramyl peptide precursors of bacterial cell wall peptidoglycan. Anal Biochem. 1981 Jun;114(1):59–63. doi: 10.1016/0003-2697(81)90451-6. [DOI] [PubMed] [Google Scholar]
- Freese E. B., Cole R. M., Klofat W., Freese E. Growth, sporulation, and enzyme defects of glucosamine mutants of Bacillus subtilis. J Bacteriol. 1970 Mar;101(3):1046–1062. doi: 10.1128/jb.101.3.1046-1062.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell E. W. Recycling of murein by Escherichia coli. J Bacteriol. 1985 Jul;163(1):305–310. doi: 10.1128/jb.163.1.305-310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hove-Jensen B. Identification of tms-26 as an allele of the gcaD gene, which encodes N-acetylglucosamine 1-phosphate uridyltransferase in Bacillus subtilis. J Bacteriol. 1992 Nov;174(21):6852–6856. doi: 10.1128/jb.174.21.6852-6856.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T. M., Stachula S. A., Raetz C. R., Anderson M. S. The firA gene of Escherichia coli encodes UDP-3-O-(R-3-hydroxymyristoyl)-glucosamine N-acyltransferase. The third step of endotoxin biosynthesis. J Biol Chem. 1993 Sep 15;268(26):19866–19874. [PubMed] [Google Scholar]
- Kuhn H. M., Meier-Dieter U., Mayer H. ECA, the enterobacterial common antigen. FEMS Microbiol Rev. 1988 Sep;4(3):195–222. doi: 10.1111/j.1574-6968.1988.tb02743.x. [DOI] [PubMed] [Google Scholar]
- Kukko-Kalske E., Lintunen M., Inen M. K., Lahti R., Heinonen J. Intracellular PPi concentration is not directly dependent on amount of inorganic pyrophosphatase in Escherichia coli K-12 cells. J Bacteriol. 1989 Aug;171(8):4498–4500. doi: 10.1128/jb.171.8.4498-4500.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mengin-Lecreulx D., Flouret B., van Heijenoort J. Cytoplasmic steps of peptidoglycan synthesis in Escherichia coli. J Bacteriol. 1982 Sep;151(3):1109–1117. doi: 10.1128/jb.151.3.1109-1117.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengin-Lecreulx D., Flouret B., van Heijenoort J. Pool levels of UDP N-acetylglucosamine and UDP N-acetylglucosamine-enolpyruvate in Escherichia coli and correlation with peptidoglycan synthesis. J Bacteriol. 1983 Jun;154(3):1284–1290. doi: 10.1128/jb.154.3.1284-1290.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengin-Lecreulx D., Parquet C., Desviat L. R., Plá J., Flouret B., Ayala J. A., van Heijenoort J. Organization of the murE-murG region of Escherichia coli: identification of the murD gene encoding the D-glutamic-acid-adding enzyme. J Bacteriol. 1989 Nov;171(11):6126–6134. doi: 10.1128/jb.171.11.6126-6134.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengin-Lecreulx D., van Heijenoort J. Correlation between the effects of fosfomycin and chloramphenicol on Escherichia coli. FEMS Microbiol Lett. 1990 Jan 1;54(1-3):129–133. doi: 10.1016/0378-1097(90)90270-z. [DOI] [PubMed] [Google Scholar]
- Mengin-Lecreulx D., van Heijenoort J. Effect of growth conditions on peptidoglycan content and cytoplasmic steps of its biosynthesis in Escherichia coli. J Bacteriol. 1985 Jul;163(1):208–212. doi: 10.1128/jb.163.1.208-212.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengin-Lecreulx D., van Heijenoort J. Identification of the glmU gene encoding N-acetylglucosamine-1-phosphate uridyltransferase in Escherichia coli. J Bacteriol. 1993 Oct;175(19):6150–6157. doi: 10.1128/jb.175.19.6150-6157.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud C., Blanot D., Flouret B., Van Heijenoort J. Partial purification and specificity studies of the D-glutamate-adding and D-alanyl-D-alanine-adding enzymes from Escherichia coli K12. Eur J Biochem. 1987 Aug 3;166(3):631–637. doi: 10.1111/j.1432-1033.1987.tb13560.x. [DOI] [PubMed] [Google Scholar]
- Nilsson D., Hove-Jensen B., Arnvig K. Primary structure of the tms and prs genes of Bacillus subtilis. Mol Gen Genet. 1989 Sep;218(3):565–571. doi: 10.1007/BF00332425. [DOI] [PubMed] [Google Scholar]
- Plumbridge J. A., Cochet O., Souza J. M., Altamirano M. M., Calcagno M. L., Badet B. Coordinated regulation of amino sugar-synthesizing and -degrading enzymes in Escherichia coli K-12. J Bacteriol. 1993 Aug;175(16):4951–4956. doi: 10.1128/jb.175.16.4951-4956.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfield L., Pearlman-Kothencz M. Synthesis and assembly of bacterial membrane components. A lipopolysaccharide-phospholipid-protein complex excreted by living bacteria. J Mol Biol. 1969 Sep 28;44(3):477–492. doi: 10.1016/0022-2836(69)90374-x. [DOI] [PubMed] [Google Scholar]
- STROMINGER J. L., SMITH M. S. Uridine diphosphoacetylglucosamine pyrophosphorylase. J Biol Chem. 1959 Jul;234(7):1822–1827. [PubMed] [Google Scholar]
- Sarvas M. Mutant of Escherichia coli K-12 defective in D-glucosamine biosynthesis. J Bacteriol. 1971 Feb;105(2):467–471. doi: 10.1128/jb.105.2.467-471.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M. Eight bacterial proteins, including UDP-N-acetylglucosamine acyltransferase (LpxA) and three other transferases of Escherichia coli, consist of a six-residue periodicity theme. FEMS Microbiol Lett. 1992 Oct 15;76(3):249–254. doi: 10.1016/0378-1097(92)90344-n. [DOI] [PubMed] [Google Scholar]
- Vuorio R., Hirvas L., Vaara M. The Ssc protein of enteric bacteria has significant homology to the acyltransferase Lpxa of lipid A biosynthesis, and to three acetyltransferases. FEBS Lett. 1991 Nov 4;292(1-2):90–94. doi: 10.1016/0014-5793(91)80841-p. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Gay N. J., Saraste M., Eberle A. N. DNA sequence around the Escherichia coli unc operon. Completion of the sequence of a 17 kilobase segment containing asnA, oriC, unc, glmS and phoS. Biochem J. 1984 Dec 15;224(3):799–815. doi: 10.1042/bj2240799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. J. Control of amino sugar metabolism in Escherichia coli and isolation of mutants unable to degrade amino sugars. Biochem J. 1968 Feb;106(4):847–858. doi: 10.1042/bj1060847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C., Wu T. C. Isolation and characterization of a glucosamine-requiring mutant of Escherichia coli K-12 defective in glucosamine-6-phosphate synthetase. J Bacteriol. 1971 Feb;105(2):455–466. doi: 10.1128/jb.105.2.455-466.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]



