Abstract
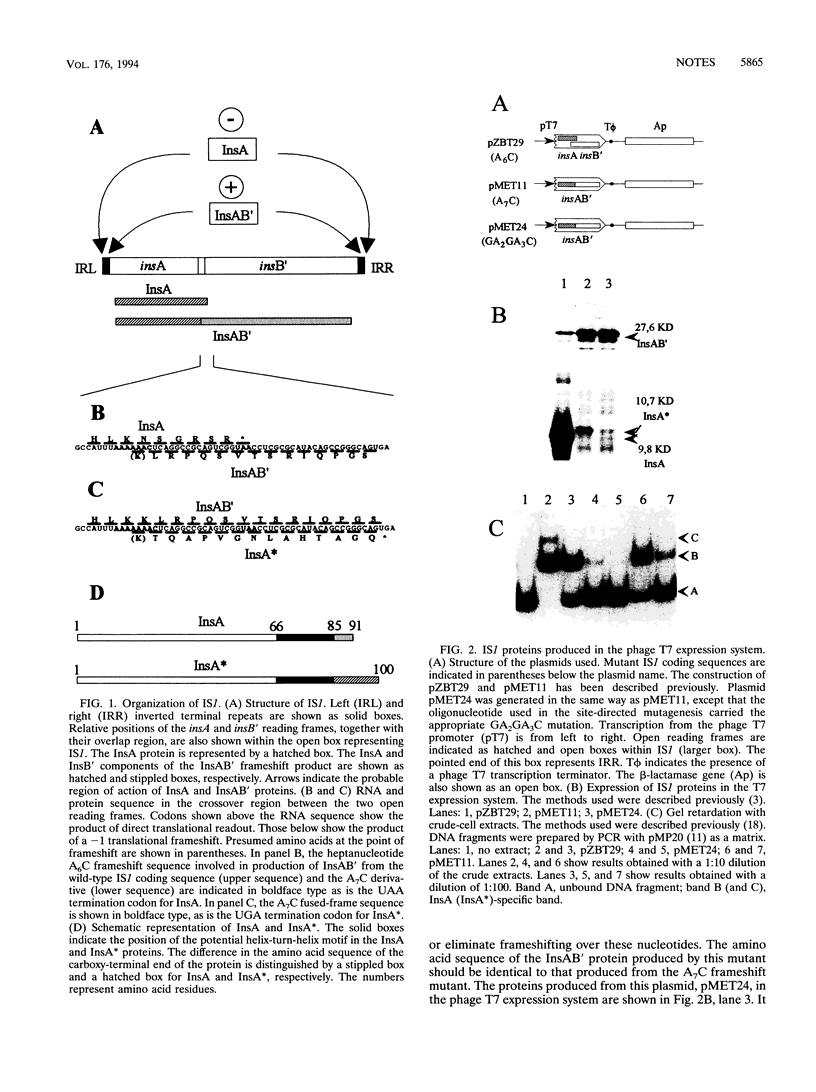
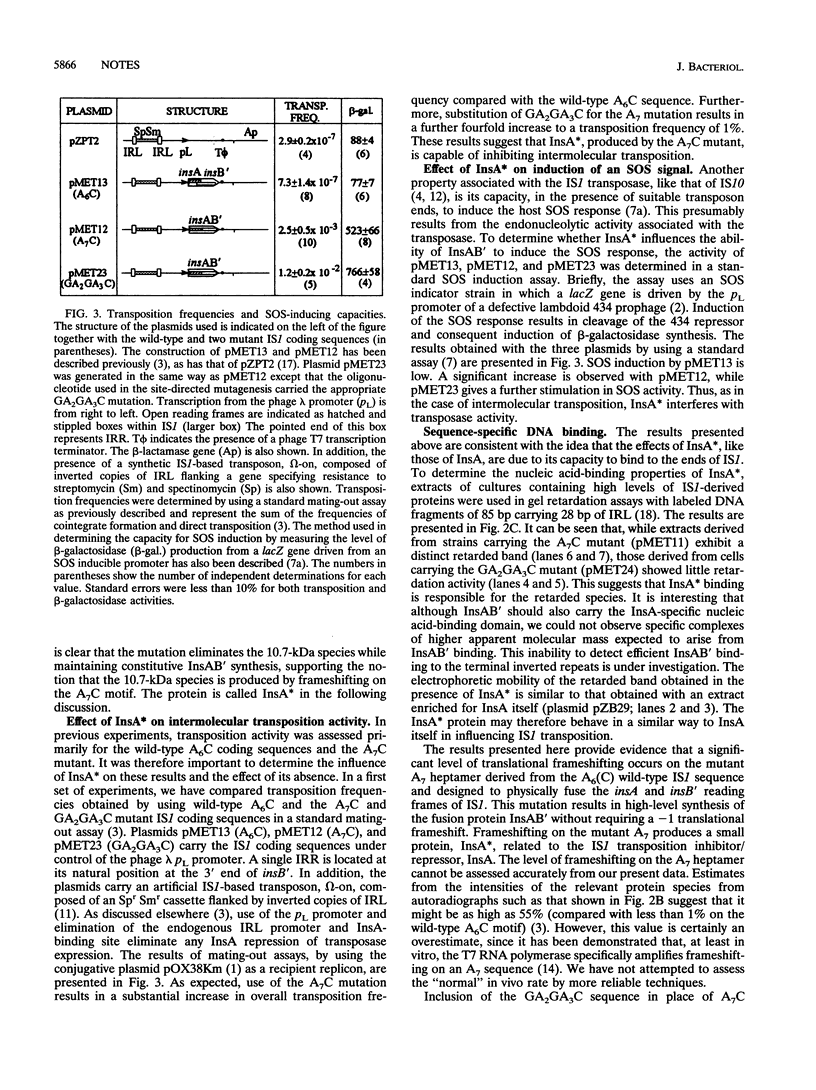
The transposase of the bacterial insertion sequence IS1 is normally expressed by inefficient translational frameshifting between an upstream reading frame which itself specifies a transposition inhibitor, InsA, and a second consecutive reading frame located immediately downstream. A fused-frame mutant which carries an additional base pair inserted at the point of frameshifting was constructed. This mutant exhibits high transposition activity and should express the transposase, InsAB', constitutively without frameshifting. Unexpectedly, a second protein species was observed to be expressed from this mutant. We demonstrate here that this protein, InsA*, results from continued frameshifting on the modified frameshift motif. The protein retains the activities of the repressor InsA. Its elimination, by further modification of the frameshift motif, results in a further increase in various transposition activities of IS1. These results support the hypothesis that a single IS1-encoded protein, InsAB', is necessary for transposition.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chandler M., Galas D. J. Cointegrate formation mediated by Tn9. II. Activity of IS1 is modulated by external DNA sequences. J Mol Biol. 1983 Oct 15;170(1):61–91. doi: 10.1016/s0022-2836(83)80227-7. [DOI] [PubMed] [Google Scholar]
- Elespuru R. K., Yarmolinsky M. B. A colorimetric assay of lysogenic induction designed for screening potential carcinogenic and carcinostatic agents. Environ Mutagen. 1979;1(1):65–78. doi: 10.1002/em.2860010113. [DOI] [PubMed] [Google Scholar]
- Escoubas J. M., Prère M. F., Fayet O., Salvignol I., Galas D., Zerbib D., Chandler M. Translational control of transposition activity of the bacterial insertion sequence IS1. EMBO J. 1991 Mar;10(3):705–712. doi: 10.1002/j.1460-2075.1991.tb08000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haniford D. B., Chelouche A. R., Kleckner N. A specific class of IS10 transposase mutants are blocked for target site interactions and promote formation of an excised transposon fragment. Cell. 1989 Oct 20;59(2):385–394. doi: 10.1016/0092-8674(89)90299-7. [DOI] [PubMed] [Google Scholar]
- Jacks T., Power M. D., Masiarz F. R., Luciw P. A., Barr P. J., Varmus H. E. Characterization of ribosomal frameshifting in HIV-1 gag-pol expression. Nature. 1988 Jan 21;331(6153):280–283. doi: 10.1038/331280a0. [DOI] [PubMed] [Google Scholar]
- Jacks T., Townsley K., Varmus H. E., Majors J. Two efficient ribosomal frameshifting events are required for synthesis of mouse mammary tumor virus gag-related polyproteins. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4298–4302. doi: 10.1073/pnas.84.12.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakowec M., Prentki P., Chandler M., Galas D. J. Mutational analysis of the open reading frames in the transposable element IS1. Genetics. 1988 Sep;120(1):47–55. doi: 10.1093/genetics/120.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida C., Machida Y. Regulation of IS1 transposition by the insA gene product. J Mol Biol. 1989 Aug 20;208(4):567–574. doi: 10.1016/0022-2836(89)90148-4. [DOI] [PubMed] [Google Scholar]
- Machida Y., Machida C., Ohtsubo E. Insertion element IS1 encodes two structural genes required for its transposition. J Mol Biol. 1984 Aug 5;177(2):229–245. doi: 10.1016/0022-2836(84)90454-6. [DOI] [PubMed] [Google Scholar]
- Nyman K., Ohtsubo H., Davison D., Ohtsubo E. Distribution of insertion element IS1 in natural isolates of Escherichia coli. Mol Gen Genet. 1983;189(3):516–518. doi: 10.1007/BF00325920. [DOI] [PubMed] [Google Scholar]
- Prentki P., Pham M. H., Gamas P., Chandler M., Galas D. J. Artificial transposable elements in the study of the ends of IS1. Gene. 1987;61(1):91–101. doi: 10.1016/0378-1119(87)90368-4. [DOI] [PubMed] [Google Scholar]
- Roberts D., Kleckner N. Tn10 transposition promotes RecA-dependent induction of a lambda prophage. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6037–6041. doi: 10.1073/pnas.85.16.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y., Ohtsubo E. Frameshifting is required for production of the transposase encoded by insertion sequence 1. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4609–4613. doi: 10.1073/pnas.86.12.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchihashi Z., Brown P. O. Sequence requirements for efficient translational frameshifting in the Escherichia coli dnaX gene and the role of an unstable interaction between tRNA(Lys) and an AAG lysine codon. Genes Dev. 1992 Mar;6(3):511–519. doi: 10.1101/gad.6.3.511. [DOI] [PubMed] [Google Scholar]
- Weiss R. B., Dunn D. M., Shuh M., Atkins J. F., Gesteland R. F. E. coli ribosomes re-phase on retroviral frameshift signals at rates ranging from 2 to 50 percent. New Biol. 1989 Nov;1(2):159–169. [PubMed] [Google Scholar]
- Zerbib D., Jakowec M., Prentki P., Galas D. J., Chandler M. Expression of proteins essential for IS1 transposition: specific binding of InsA to the ends of IS1. EMBO J. 1987 Oct;6(10):3163–3169. doi: 10.1002/j.1460-2075.1987.tb02627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbib D., Polard P., Escoubas J. M., Galas D., Chandler M. The regulatory role of the IS1-encoded InsA protein in transposition. Mol Microbiol. 1990 Mar;4(3):471–477. doi: 10.1111/j.1365-2958.1990.tb00613.x. [DOI] [PubMed] [Google Scholar]
- Zerbib D., Prentki P., Gamas P., Freund E., Galas D. J., Chandler M. Functional organization of the ends of IS1: specific binding site for an IS 1-encoded protein. Mol Microbiol. 1990 Sep;4(9):1477–1486. [PubMed] [Google Scholar]