Abstract
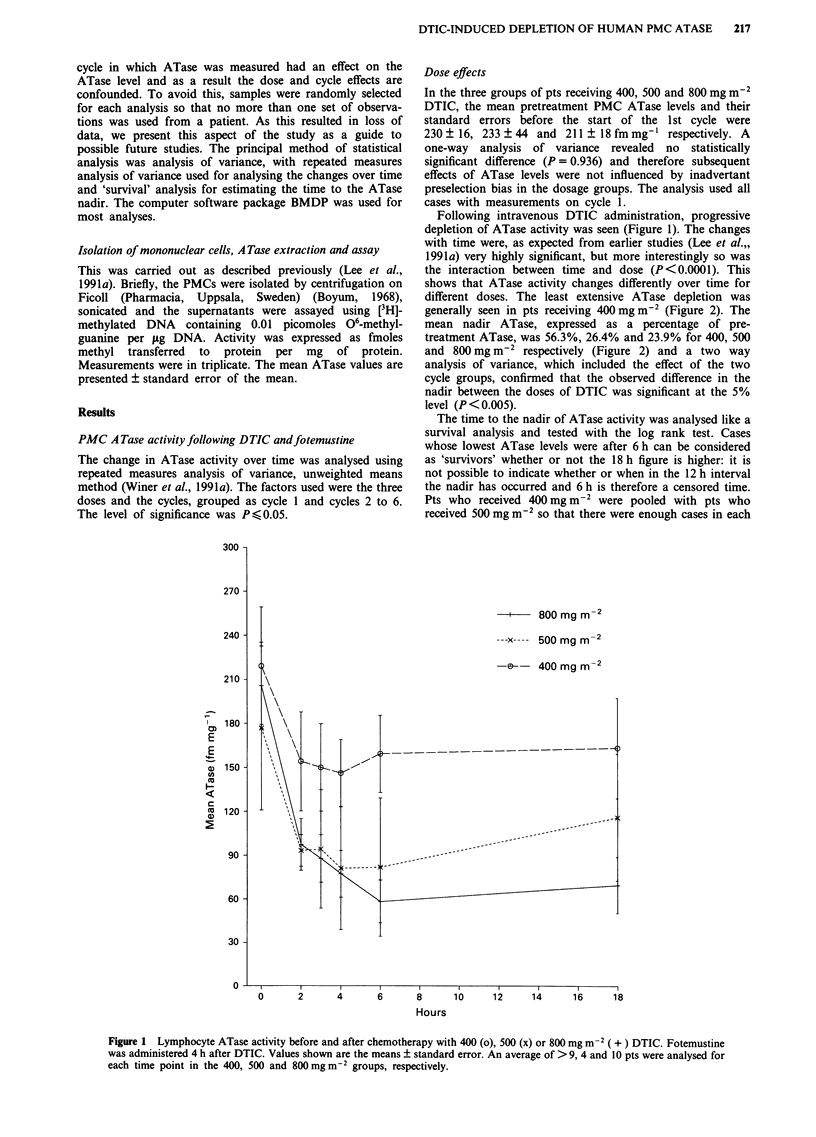
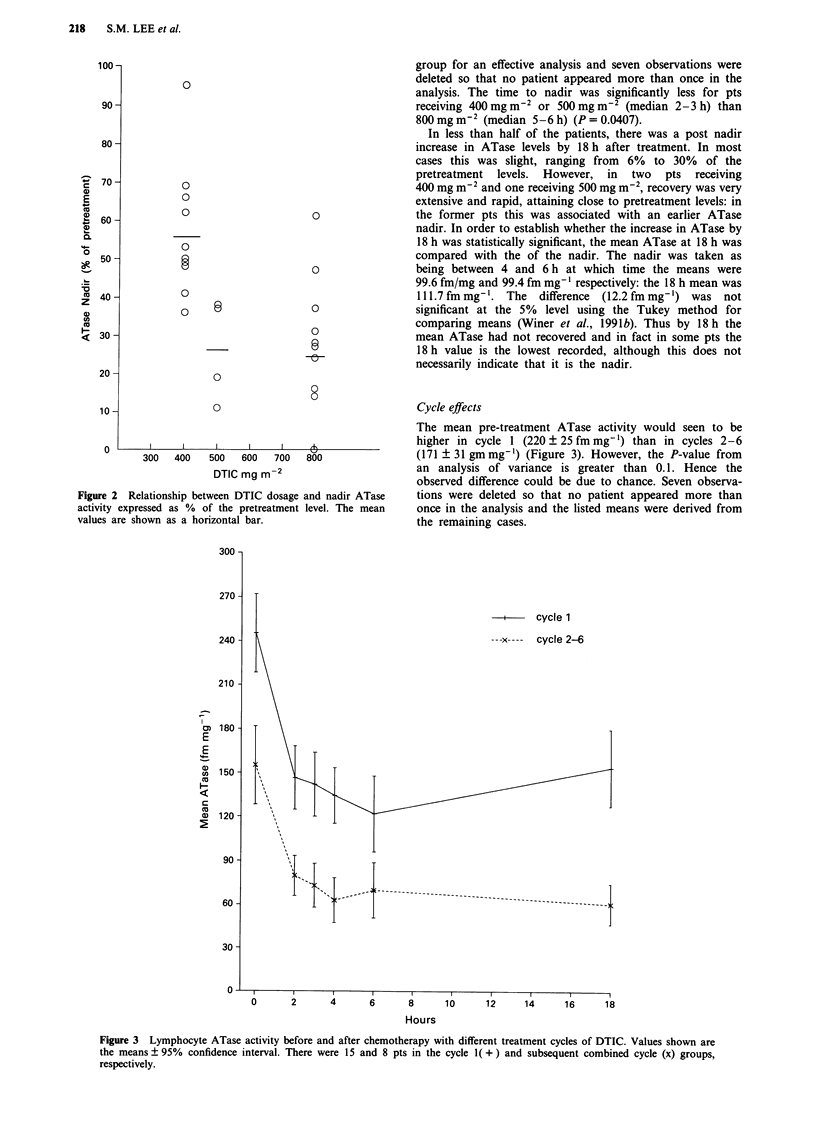
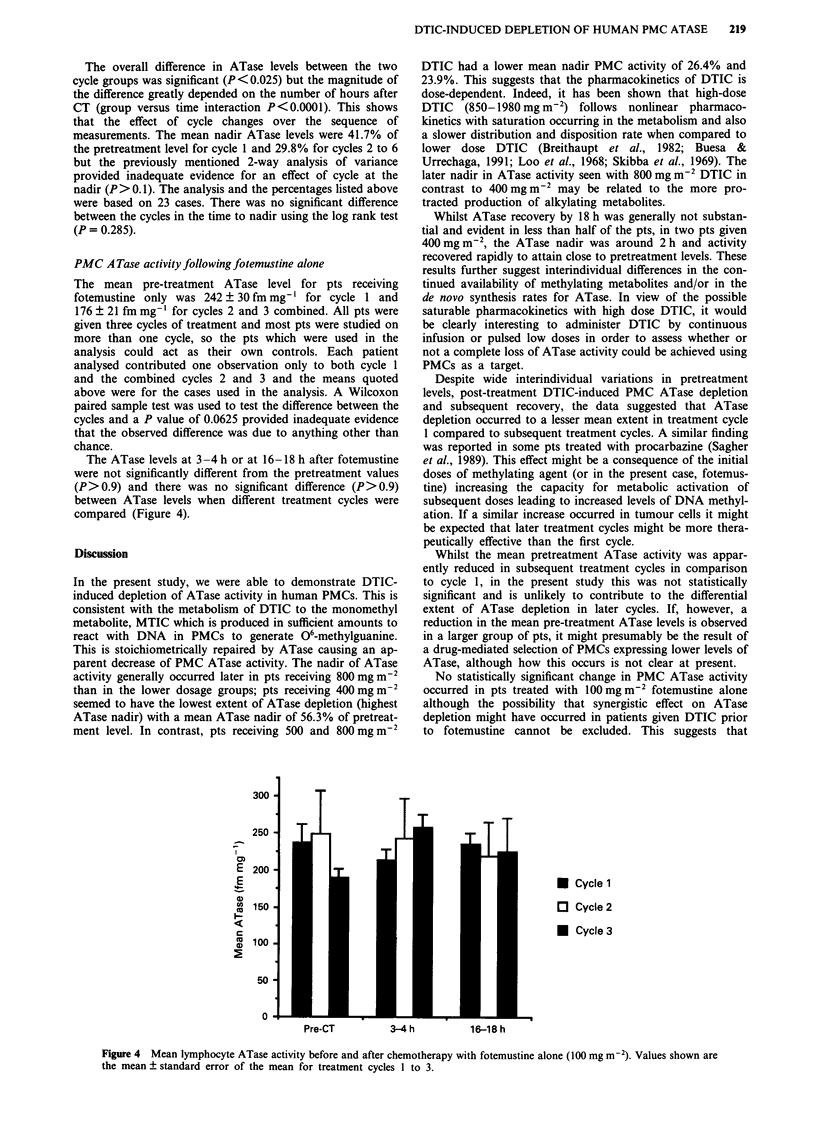
There is increasing experimental evidence to suggest that endogenous expression of O6-alkylguanine-DNA-alkyltransferase (ATase) is a major factor in cellular resistance to certain chemotherapeutic agents including dacarbazine (DTIC). We have recently shown wide interindividual variation in the depletion and subsequent regeneration of ATase in peripheral blood mononuclear cells (PMCs) following DTIC and this has now been extended to ascertain whether or not depletion is related to dosage of DTIC used and repeated treatment cycles of chemotherapy. ATase levels were measured in three groups of 25 patients (pts) up to 24 h after receiving DTIC at 400 mg m-2, 500 mg m-2 or 800 mg m-2. Each group also received fotemustine (100 mg m-2), 4 h after DTIC. The lowest extent of ATase depletion (highest nadir ATase) was seen in patients receiving 400 mg m-2. The mean nadir ATase, expressed as a percentage of pre-treatment ATase, was respectively 56.3%, 26.4% and 23.9% for 400 mg m-2, 500 mg m-2 and 800 mg m-2. The median nadir of ATase activity for pts receiving 800 mg m-2 pts was at 4-6 h and for pts given lower doses it was at 2-3 h. In addition, repeated measures analysis of variance of observations before chemotherapy, then at 2, 3, 4, 6 and 18 h after chemotherapy provides some evidence that ATase was depleted to a lesser extent after cycle 1 than after subsequent cycles (P = 0.025). It also provides evidence that the change in ATase activity over time varied with dose and cycle. The findings can be interpreted on the basis of a dosage-dependent metabolism of DTIC to an agent capable of methylation of DNA and subsequent depletion of PMC ATase: with higher DTIC doses, the extent of ATase depletion may be limited by the pharmacokinetics of DTIC metabolism. PMC ATase was measured in another group of 8 pts at various times after receiving only fotemustine (100 mg m-2) and in contrast to DTIC, no ATase depletion was seen suggesting that insufficient concentrations of fotemustine and/or its metabolites were available to react with DNA to produce a depletion of PMC ATase activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breithaupt H., Dammann A., Aigner K. Pharmacokinetics of dacarbazine (DTIC) and its metabolite 5-aminoimidazole-4-carboxamide (AIC) following different dose schedules. Cancer Chemother Pharmacol. 1982;9(2):103–109. doi: 10.1007/BF00265388. [DOI] [PubMed] [Google Scholar]
- Brennand J., Margison G. P. Reduction of the toxicity and mutagenicity of alkylating agents in mammalian cells harboring the Escherichia coli alkyltransferase gene. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6292–6296. doi: 10.1073/pnas.83.17.6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buesa J. M., Urréchaga E. Clinical pharmacokinetics of high-dose DTIC. Cancer Chemother Pharmacol. 1991;28(6):475–479. doi: 10.1007/BF00685826. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Catapano C. V., Broggini M., Erba E., Ponti M., Mariani L., Citti L., D'Incalci M. In vitro and in vivo methazolastone-induced DNA damage and repair in L-1210 leukemia sensitive and resistant to chloroethylnitrosoureas. Cancer Res. 1987 Sep 15;47(18):4884–4889. [PubMed] [Google Scholar]
- Comis R. L. DTIC (NSC-45388) in malignant melanoma: a perspective. Cancer Treat Rep. 1976 Feb;60(2):165–176. [PubMed] [Google Scholar]
- Cowan D. H., Bergsagel D. E. Intermittent treatment of metastatic malignant melanoma with high-dose 5-(3,3-dimethyl-1-triazeno)imidazole-4-carboxamide (NSC-45388). Cancer Chemother Rep. 1971 Apr;55(2):175–181. [PubMed] [Google Scholar]
- D'Incalci M., Citti L., Taverna P., Catapano C. V. Importance of the DNA repair enzyme O6-alkyl guanine alkyltransferase (AT) in cancer chemotherapy. Cancer Treat Rev. 1988 Dec;15(4):279–292. doi: 10.1016/0305-7372(88)90026-6. [DOI] [PubMed] [Google Scholar]
- Dolan M. E., Corsico C. D., Pegg A. E. Exposure of HeLa cells to 0(6)-alkylguanines increases sensitivity to the cytotoxic effects of alkylating agents. Biochem Biophys Res Commun. 1985 Oct 15;132(1):178–185. doi: 10.1016/0006-291x(85)91004-6. [DOI] [PubMed] [Google Scholar]
- Dolan M. E., Mitchell R. B., Mummert C., Moschel R. C., Pegg A. E. Effect of O6-benzylguanine analogues on sensitivity of human tumor cells to the cytotoxic effects of alkylating agents. Cancer Res. 1991 Jul 1;51(13):3367–3372. [PubMed] [Google Scholar]
- Dumenco L. L., Warman B., Hatzoglou M., Lim I. K., Abboud S. L., Gerson S. L. Increase in nitrosourea resistance in mammalian cells by retrovirally mediated gene transfer of bacterial O6-alkylguanine-DNA alkyltransferase. Cancer Res. 1989 Nov 1;49(21):6044–6051. [PubMed] [Google Scholar]
- Gerson S. L. Modulation of human lymphocyte O6-alkylguanine-DNA alkyltransferase by streptozotocin in vivo. Cancer Res. 1989 Jun 1;49(11):3134–3138. [PubMed] [Google Scholar]
- Gerson S. L., Trey J. E., Miller K., Berger N. A. Comparison of O6-alkylguanine-DNA alkyltransferase activity based on cellular DNA content in human, rat and mouse tissues. Carcinogenesis. 1986 May;7(5):745–749. doi: 10.1093/carcin/7.5.745. [DOI] [PubMed] [Google Scholar]
- Gerson S. L., Trey J. E., Miller K. Potentiation of nitrosourea cytotoxicity in human leukemic cells by inactivation of O6-alkylguanine-DNA alkyltransferase. Cancer Res. 1988 Mar 15;48(6):1521–1527. [PubMed] [Google Scholar]
- Gibson N. W., Hartley J. A., Barnes D., Erickson L. C. Combined effects of streptozotocin and mitozolomide against four human cell lines of the Mer+ phenotype. Cancer Res. 1986 Oct;46(10):4995–4998. [PubMed] [Google Scholar]
- Gibson N. W., Hartley J., La France R. J., Vaughan K. Differential cytotoxicity and DNA-damaging effects produced in human cells of the Mer+ and Mer- phenotypes by a series of alkyltriazenylimidazoles. Carcinogenesis. 1986 Feb;7(2):259–265. doi: 10.1093/carcin/7.2.259. [DOI] [PubMed] [Google Scholar]
- Hayward I. P., Parsons P. G. Comparison of virus reactivation, DNA base damage, and cell cycle effects in autologous human melanoma cells resistant to methylating agents. Cancer Res. 1984 Jan;44(1):55–58. [PubMed] [Google Scholar]
- Jacquillat C., Khayat D., Banzet P., Weil M., Fumoleau P., Avril M. F., Namer M., Bonneterre J., Kerbrat P., Bonerandi J. J. Final report of the French multicenter phase II study of the nitrosourea fotemustine in 153 evaluable patients with disseminated malignant melanoma including patients with cerebral metastases. Cancer. 1990 Nov 1;66(9):1873–1878. doi: 10.1002/1097-0142(19901101)66:9<1873::aid-cncr2820660904>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Jelinek J., Kleibl K., Dexter T. M., Margison G. P. Transfection of murine multi-potent haemopoietic stem cells with an E. coli DNA alkyltransferase gene confers resistance to the toxic effects of alkylating agents. Carcinogenesis. 1988 Jan;9(1):81–87. doi: 10.1093/carcin/9.1.81. [DOI] [PubMed] [Google Scholar]
- Kaina B., Fritz G., Mitra S., Coquerelle T. Transfection and expression of human O6-methylguanine-DNA methyltransferase (MGMT) cDNA in Chinese hamster cells: the role of MGMT in protection against the genotoxic effects of alkylating agents. Carcinogenesis. 1991 Oct;12(10):1857–1867. doi: 10.1093/carcin/12.10.1857. [DOI] [PubMed] [Google Scholar]
- Kataoka H., Hall J., Karran P. Complementation of sensitivity to alkylating agents in Escherichia coli and Chinese hamster ovary cells by expression of a cloned bacterial DNA repair gene. EMBO J. 1986 Dec 1;5(12):3195–3200. doi: 10.1002/j.1460-2075.1986.tb04629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. M., Thatcher N., Margison G. P. O6-alkylguanine-DNA alkyltransferase depletion and regeneration in human peripheral lymphocytes following dacarbazine and fotemustine. Cancer Res. 1991 Jan 15;51(2):619–623. [PubMed] [Google Scholar]
- Loo T. L., Luce J. K., Jardine J. H., Frei E., 3rd Pharmacologic studies of the antitumor agent 5-(dimethyltriazeno)imidazole-4-carboxamide. Cancer Res. 1968 Dec;28(12):2448–2453. [PubMed] [Google Scholar]
- Lunn J. M., Harris A. L. Cytotoxicity of 5-(3-methyl-1-triazeno)imidazole-4-carboxamide (MTIC) on Mer+, Mer+Rem- and Mer- cell lines: differential potentiation by 3-acetamidobenzamide. Br J Cancer. 1988 Jan;57(1):54–58. doi: 10.1038/bjc.1988.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meer L., Janzer R. C., Kleihues P., Kolar G. F. In vivo metabolism and reaction with DNA of the cytostatic agent, 5-(3,3-dimethyl-1-triazeno)imidazole-4-carboxamide (DTIC). Biochem Pharmacol. 1986 Oct 1;35(19):3243–3247. doi: 10.1016/0006-2952(86)90419-3. [DOI] [PubMed] [Google Scholar]
- Pritchard K. I., Quirt I. C., Cowan D. H., Osoba D., Kutas G. J. DTIC therapy in metastatic malignant melanoma: a simplified dose schedule. Cancer Treat Rep. 1980 Oct-Nov;64(10-11):1123–1126. [PubMed] [Google Scholar]
- Sagher D., Karrison T., Schwartz J. L., Larson R. A., Strauss B. Heterogeneity of O6-alkylguanine-DNA alkyltransferase activity in peripheral blood lymphocytes: relationship between this activity in lymphocytes and in lymphoblastoid lines from normal controls and from patients with Hodgkin's disease or non-Hodgkin's lymphoma. Cancer Res. 1989 Oct 1;49(19):5339–5344. [PubMed] [Google Scholar]
- Samson L., Derfler B., Waldstein E. A. Suppression of human DNA alkylation-repair defects by Escherichia coli DNA-repair genes. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5607–5610. doi: 10.1073/pnas.83.15.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibba J. L., Ramirez G., Beal D. D., Bryan G. T. Preliminary clinical trial and the physiologic disposition of 4(5)-(3,3-dimethyl-1-triazeno)imidazole-5(4)-carboxamide in man. Cancer Res. 1969 Nov;29(11):1944–1951. [PubMed] [Google Scholar]
- Zlotogorski C., Erickson L. C. Pretreatment of human colon tumor cells with DNA methylating agents inhibits their ability to repair chloroethyl monoadducts. Carcinogenesis. 1984 Jan;5(1):83–87. doi: 10.1093/carcin/5.1.83. [DOI] [PubMed] [Google Scholar]


