Abstract
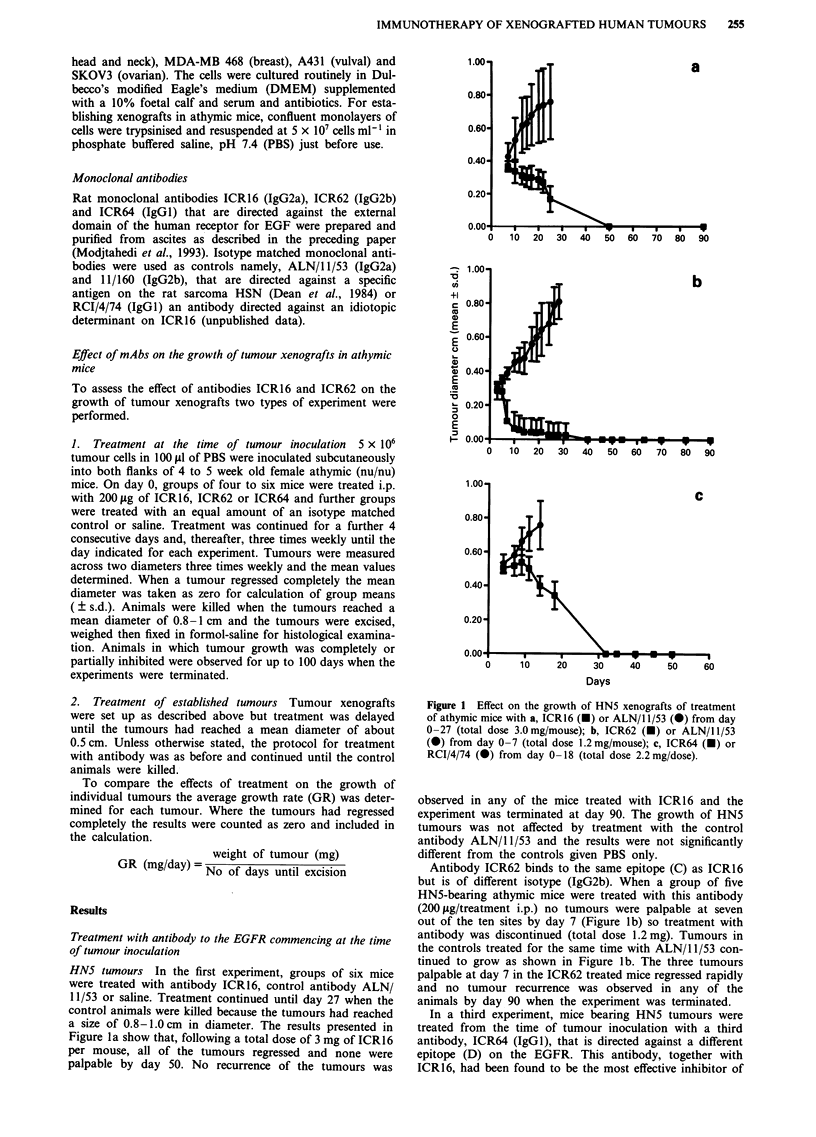
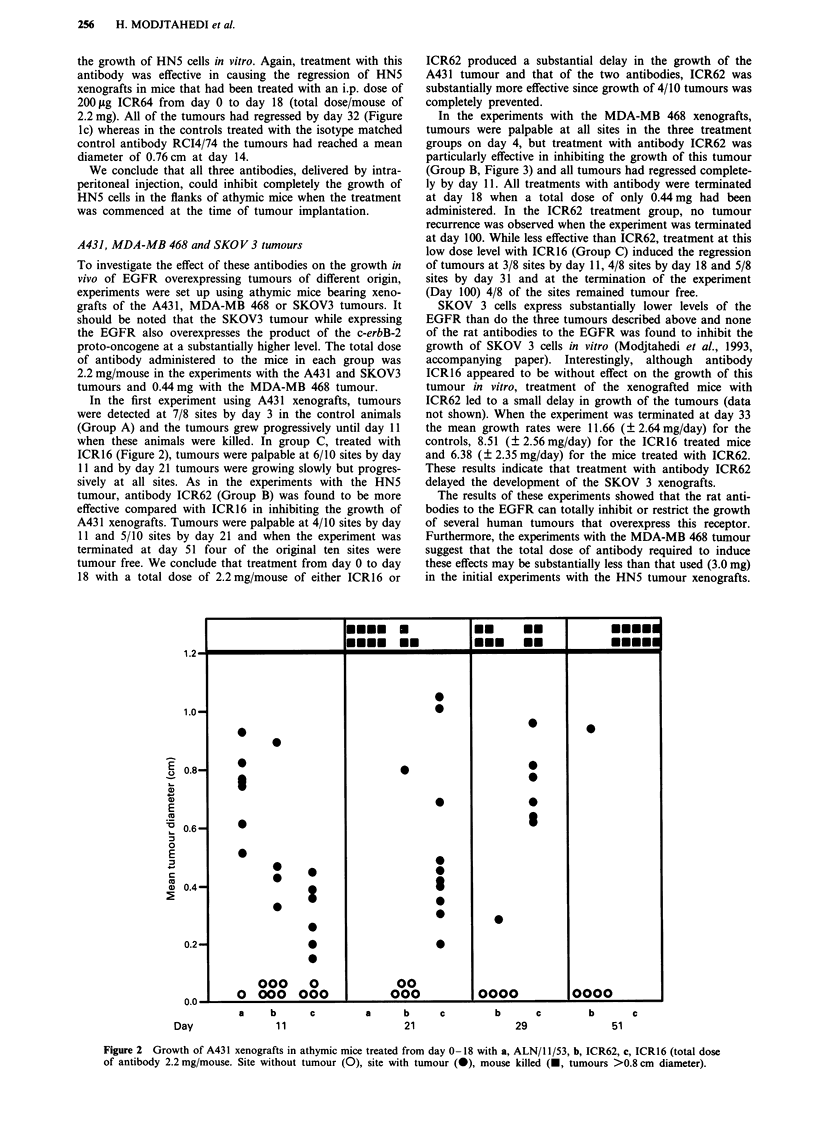
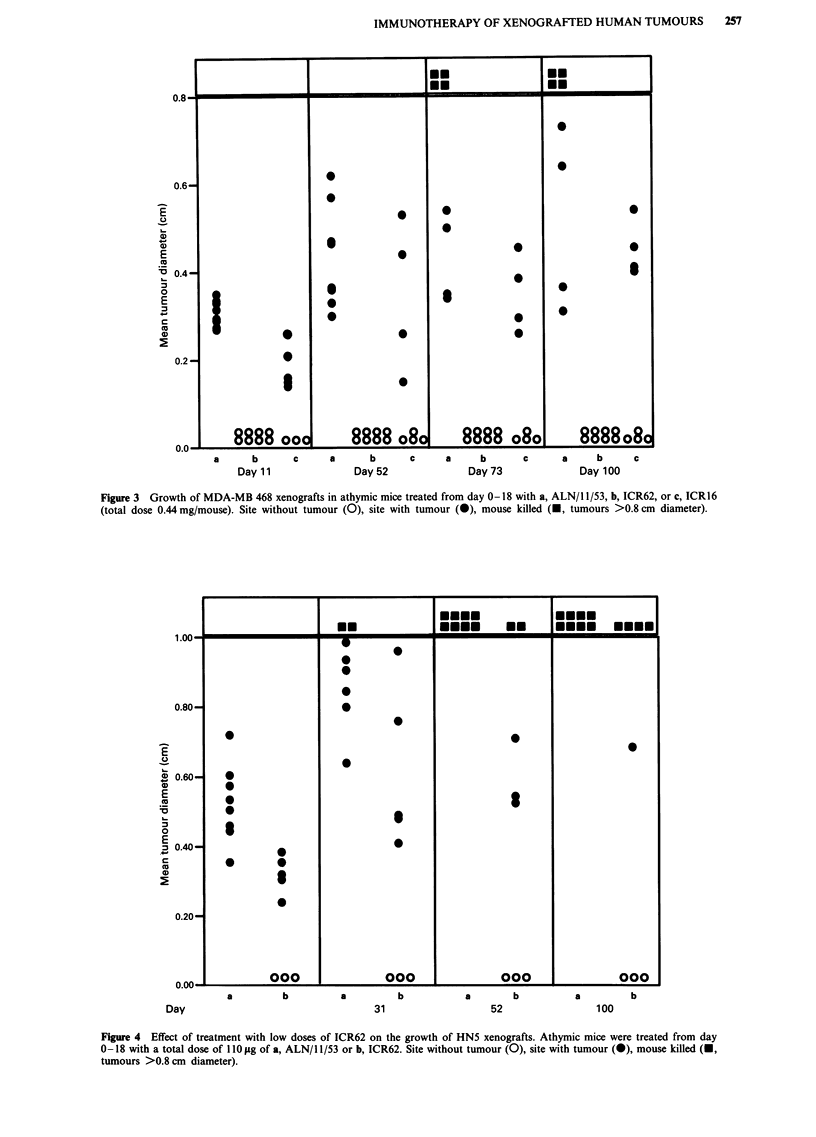
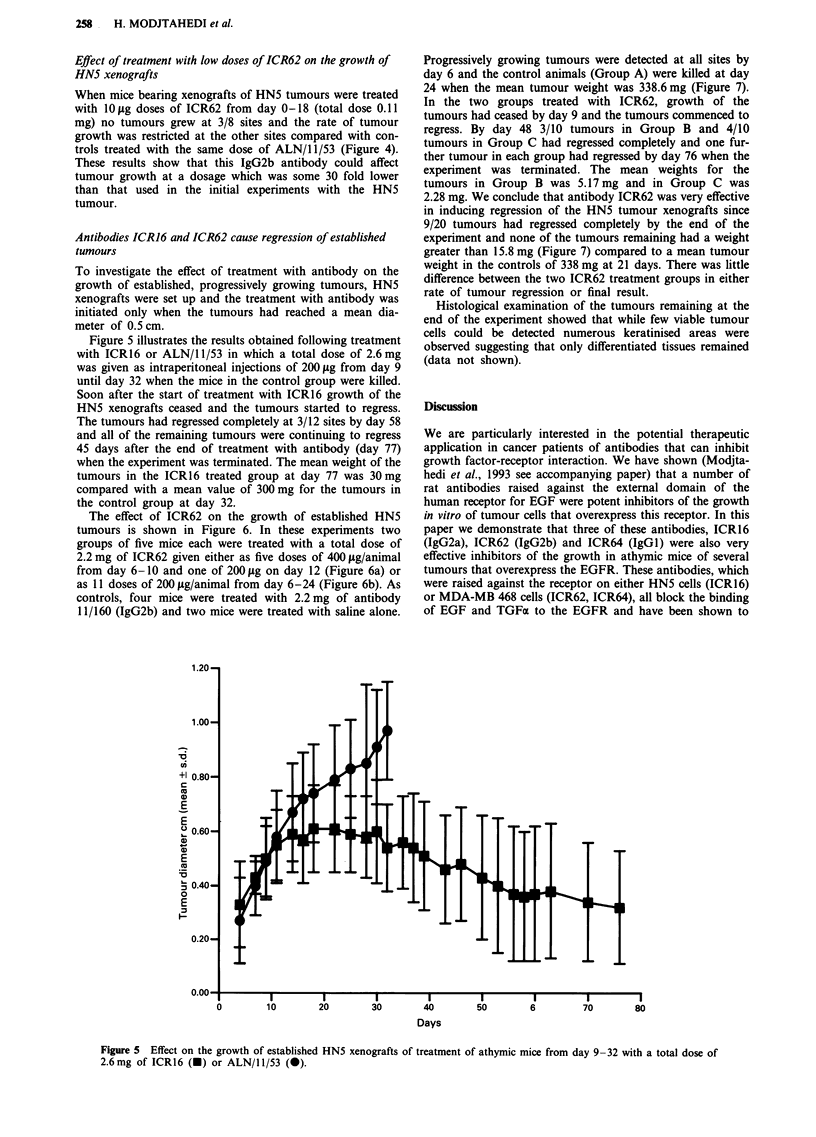
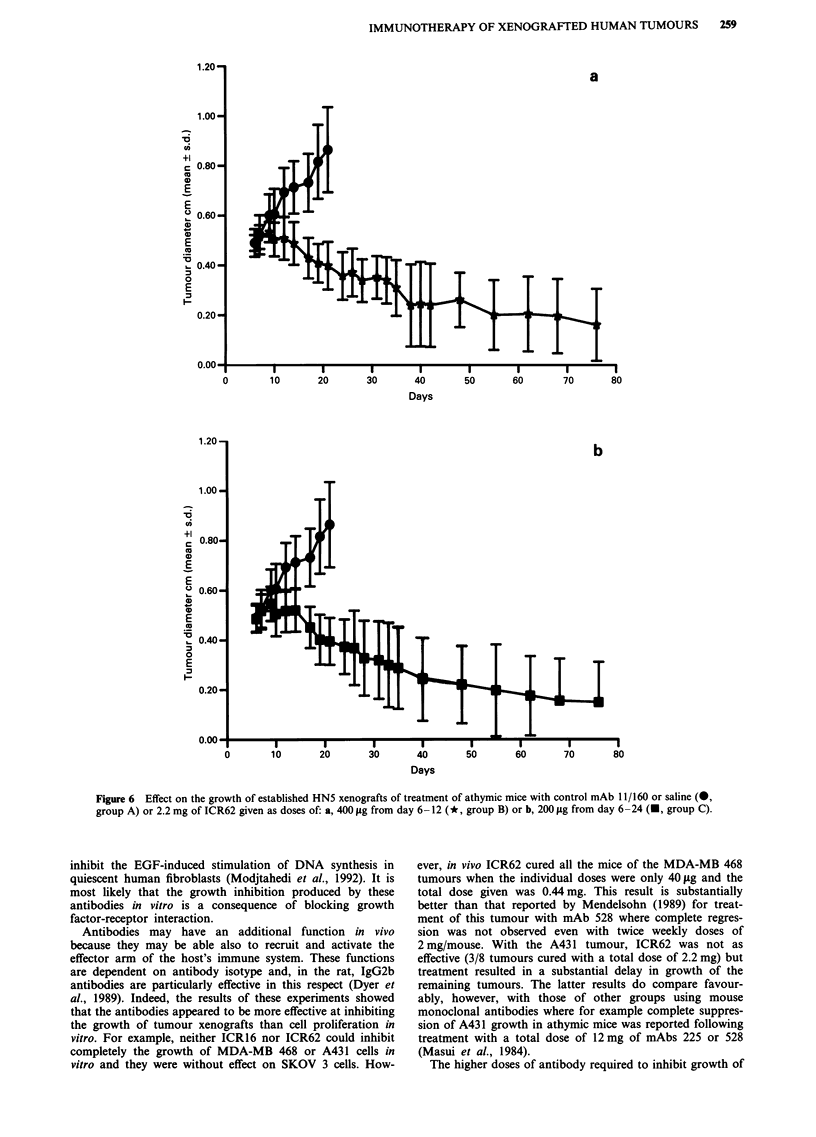
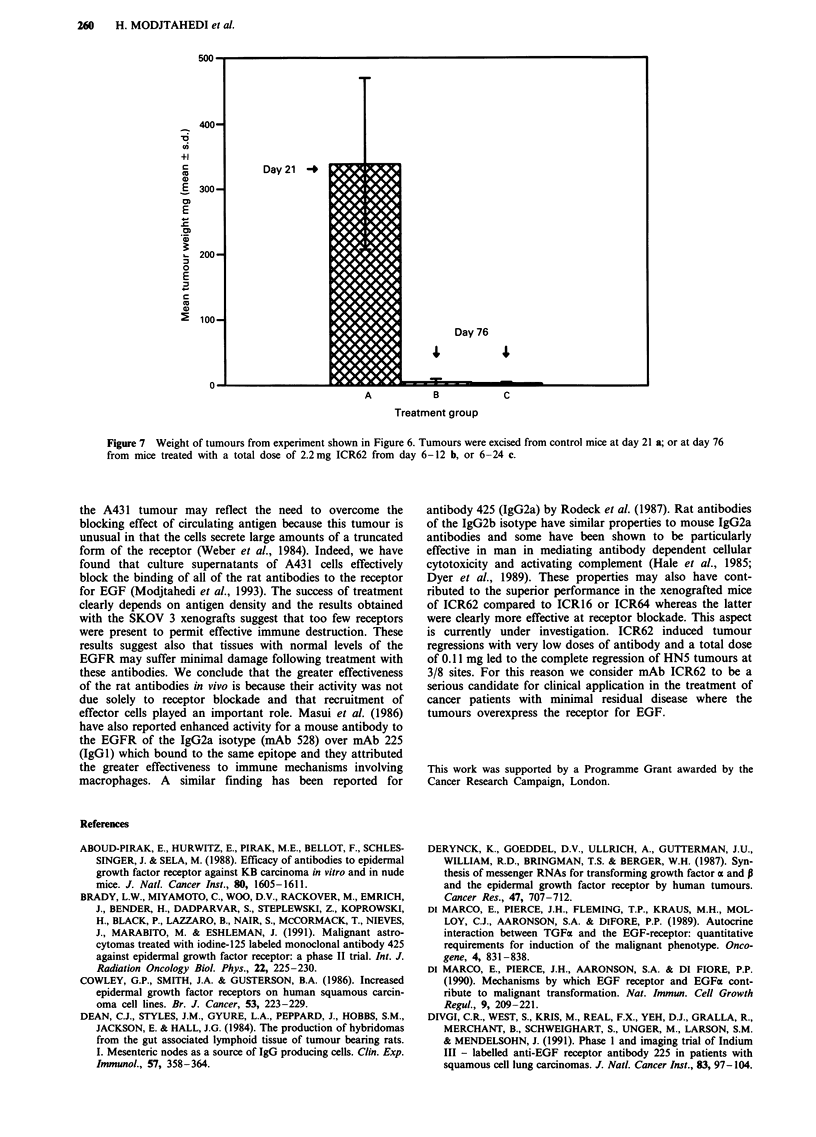
Athymic mice bearing xenografts of human tumours that overexpress the receptor (EGFR) for EGF and TGF alpha have been used to evaluate the therapeutic potential of three new rat monoclonal antibodies (mAbs) directed against two distinct epitopes on the extracellular domain of the human EGFR. The antibodies, ICR16 (IgG2a), ICR62 (IgG2b) and ICR64 (IgG1), have been shown (Modjtahedi et al., 1993) to be potent inhibitors of the growth in vitro of a number of human squamous cell carcinomas because they block receptor-ligand interaction. When given i.p. at 200 micrograms dose, the three antibodies were found to induce complete regression of xenografts of the HN5 tumour if treatment with antibody commenced at the time of tumour implantation (total doses: ICR16, 3.0 mg; ICR62, 1.2 mg; ICR64, 2.2 mg). More importantly when treatment was delayed until the tumours were established (mean diam. 0.5 cm) both ICR16 and ICR62 induced complete or almost complete regression of the tumours. Furthermore, treatment with a total dose of only 0.44 mg of ICR62 was found to induce complete remission of xenografts of the breast carcinoma MDA-MB 468, but ICR16 was less effective at this dose of antibody and only 4/8 tumours regressed completely. ICR16 and ICR62 were poor inhibitors of the growth in vitro of the vulval carcinoma A431, but both induced a substantial delay in the growth of xenografts of this tumour and 4/8 tumours regressed completely in the mice treated with ICR62 (total dose 2.2 mg). Although ICR16 and ICR64 were more effective than ICR62 as growth inhibitors in vitro, ICR62 was found to be substantially better at inducing regression of the tumour xenografts due perhaps to additional activation of host immune effector functions by the IgG2b antibody. We conclude that these antibodies may be useful therapeutic agents that can be used alone without conjugation to other cytotoxic moieties.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboud-Pirak E., Hurwitz E., Pirak M. E., Bellot F., Schlessinger J., Sela M. Efficacy of antibodies to epidermal growth factor receptor against KB carcinoma in vitro and in nude mice. J Natl Cancer Inst. 1988 Dec 21;80(20):1605–1611. doi: 10.1093/jnci/80.20.1605. [DOI] [PubMed] [Google Scholar]
- Brady L. W., Miyamoto C., Woo D. V., Rackover M., Emrich J., Bender H., Dadparvar S., Steplewski Z., Koprowski H., Black P. Malignant astrocytomas treated with iodine-125 labeled monoclonal antibody 425 against epidermal growth factor receptor: a phase II trial. Int J Radiat Oncol Biol Phys. 1992;22(1):225–230. doi: 10.1016/0360-3016(92)91009-c. [DOI] [PubMed] [Google Scholar]
- Cowley G. P., Smith J. A., Gusterson B. A. Increased EGF receptors on human squamous carcinoma cell lines. Br J Cancer. 1986 Feb;53(2):223–229. doi: 10.1038/bjc.1986.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C. J., Styles J. M., Gyure L. A., Peppard J., Hobbs S. M., Jackson E., Hall J. G. The production of hybridomas from the gut associated lymphoid tissue of tumour bearing rats. I. Mesenteric nodes as a source of IgG producing cells. Clin Exp Immunol. 1984 Aug;57(2):358–364. [PMC free article] [PubMed] [Google Scholar]
- Derynck R., Goeddel D. V., Ullrich A., Gutterman J. U., Williams R. D., Bringman T. S., Berger W. H. Synthesis of messenger RNAs for transforming growth factors alpha and beta and the epidermal growth factor receptor by human tumors. Cancer Res. 1987 Feb 1;47(3):707–712. [PubMed] [Google Scholar]
- Di Marco E., Pierce J. H., Aaronson S. A., Di Fiore P. P. Mechanisms by which EGF receptor and TGF alpha contribute to malignant transformation. Nat Immun Cell Growth Regul. 1990;9(3):209–221. [PubMed] [Google Scholar]
- Di Marco E., Pierce J. H., Fleming T. P., Kraus M. H., Molloy C. J., Aaronson S. A., Di Fiore P. P. Autocrine interaction between TGF alpha and the EGF-receptor: quantitative requirements for induction of the malignant phenotype. Oncogene. 1989 Jul;4(7):831–838. [PubMed] [Google Scholar]
- Divgi C. R., Welt S., Kris M., Real F. X., Yeh S. D., Gralla R., Merchant B., Schweighart S., Unger M., Larson S. M. Phase I and imaging trial of indium 111-labeled anti-epidermal growth factor receptor monoclonal antibody 225 in patients with squamous cell lung carcinoma. J Natl Cancer Inst. 1991 Jan 16;83(2):97–104. doi: 10.1093/jnci/83.2.97. [DOI] [PubMed] [Google Scholar]
- Dyer M. J., Hale G., Hayhoe F. G., Waldmann H. Effects of CAMPATH-1 antibodies in vivo in patients with lymphoid malignancies: influence of antibody isotype. Blood. 1989 May 1;73(6):1431–1439. [PubMed] [Google Scholar]
- Easty D. M., Easty G. C., Carter R. L., Monaghan P., Butler L. J. Ten human carcinoma cell lines derived from squamous carcinomas of the head and neck. Br J Cancer. 1981 Jun;43(6):772–785. doi: 10.1038/bjc.1981.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis B. W., Lippman M. E., Dickson R. B. The EGF receptor system as a target for antitumor therapy. Cancer Invest. 1991;9(5):553–562. doi: 10.3109/07357909109018953. [DOI] [PubMed] [Google Scholar]
- Fendly B. M., Winget M., Hudziak R. M., Lipari M. T., Napier M. A., Ullrich A. Characterization of murine monoclonal antibodies reactive to either the human epidermal growth factor receptor or HER2/neu gene product. Cancer Res. 1990 Mar 1;50(5):1550–1558. [PubMed] [Google Scholar]
- Filmus J., Pollak M. N., Cailleau R., Buick R. N. MDA-468, a human breast cancer cell line with a high number of epidermal growth factor (EGF) receptors, has an amplified EGF receptor gene and is growth inhibited by EGF. Biochem Biophys Res Commun. 1985 Apr 30;128(2):898–905. doi: 10.1016/0006-291x(85)90131-7. [DOI] [PubMed] [Google Scholar]
- Gullick W. J. Prevalence of aberrant expression of the epidermal growth factor receptor in human cancers. Br Med Bull. 1991 Jan;47(1):87–98. doi: 10.1093/oxfordjournals.bmb.a072464. [DOI] [PubMed] [Google Scholar]
- Hale G., Clark M., Waldmann H. Therapeutic potential of rat monoclonal antibodies: isotype specificity of antibody-dependent cell-mediated cytotoxicity with human lymphocytes. J Immunol. 1985 May;134(5):3056–3061. [PubMed] [Google Scholar]
- Harris A. L. The epidermal growth factor receptor as a target for therapy. Cancer Cells. 1990 Oct;2(10):321–323. [PubMed] [Google Scholar]
- Kalofonos H. P., Pawlikowska T. R., Hemingway A., Courtenay-Luck N., Dhokia B., Snook D., Sivolapenko G. B., Hooker G. R., McKenzie C. G., Lavender P. J. Antibody guided diagnosis and therapy of brain gliomas using radiolabeled monoclonal antibodies against epidermal growth factor receptor and placental alkaline phosphatase. J Nucl Med. 1989 Oct;30(10):1636–1645. [PubMed] [Google Scholar]
- Kurachi H., Morishige K., Amemiya K., Adachi H., Hirota K., Miyake A., Tanizawa O. Importance of transforming growth factor alpha/epidermal growth factor receptor autocrine growth mechanism in an ovarian cancer cell line in vivo. Cancer Res. 1991 Nov 1;51(21):5956–5959. [PubMed] [Google Scholar]
- Masui H., Kawamoto T., Sato J. D., Wolf B., Sato G., Mendelsohn J. Growth inhibition of human tumor cells in athymic mice by anti-epidermal growth factor receptor monoclonal antibodies. Cancer Res. 1984 Mar;44(3):1002–1007. [PubMed] [Google Scholar]
- Masui H., Moroyama T., Mendelsohn J. Mechanism of antitumor activity in mice for anti-epidermal growth factor receptor monoclonal antibodies with different isotypes. Cancer Res. 1986 Nov;46(11):5592–5598. [PubMed] [Google Scholar]
- Modjtahedi H., Styles J. M., Dean C. J. The human EGF receptor as a target for cancer therapy: six new rat mAbs against the receptor on the breast carcinoma MDA-MB 468. Br J Cancer. 1993 Feb;67(2):247–253. doi: 10.1038/bjc.1993.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini R., Centis F., Martignone S., Mastroianni A., Tagliabue E., Tosi E., Ménard S., Colnaghi M. I. Characterization of a monoclonal antibody directed against the epidermal growth factor receptor binding site. Cancer Immunol Immunother. 1991;34(1):37–42. doi: 10.1007/BF01741322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeck U., Herlyn M., Herlyn D., Molthoff C., Atkinson B., Varello M., Steplewski Z., Koprowski H. Tumor growth modulation by a monoclonal antibody to the epidermal growth factor receptor: immunologically mediated and effector cell-independent effects. Cancer Res. 1987 Jul 15;47(14):3692–3696. [PubMed] [Google Scholar]
- Santon J. B., Cronin M. T., MacLeod C. L., Mendelsohn J., Masui H., Gill G. N. Effects of epidermal growth factor receptor concentration on tumorigenicity of A431 cells in nude mice. Cancer Res. 1986 Sep;46(9):4701–4705. [PubMed] [Google Scholar]
- Sato J. D., Kawamoto T., Le A. D., Mendelsohn J., Polikoff J., Sato G. H. Biological effects in vitro of monoclonal antibodies to human epidermal growth factor receptors. Mol Biol Med. 1983 Dec;1(5):511–529. [PubMed] [Google Scholar]
- Schreiber A. B., Lax I., Yarden Y., Eshhar Z., Schlessinger J. Monoclonal antibodies against receptor for epidermal growth factor induce early and delayed effects of epidermal growth factor. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7535–7539. doi: 10.1073/pnas.78.12.7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soo K. C., Ward M., Roberts K. R., Keeling F., Carter R. L., McCready V. R., Ott R. J., Powell E., Ozanne B., Westwood J. H. Radioimmunoscintigraphy of squamous carcinomas of the head and neck. Head Neck Surg. 1987 Jul-Aug;9(6):349–352. doi: 10.1002/hed.2890090608. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Autocrine growth factors and cancer. 1985 Feb 28-Mar 6Nature. 313(6005):745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Todaro G. J. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980 Oct 9;303(15):878–880. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- Steck P. A., Lee P., Hung M. C., Yung W. K. Expression of an altered epidermal growth factor receptor by human glioblastoma cells. Cancer Res. 1988 Oct 1;48(19):5433–5439. [PubMed] [Google Scholar]
- Velu T. J. Structure, function and transforming potential of the epidermal growth factor receptor. Mol Cell Endocrinol. 1990 May 7;70(3):205–216. doi: 10.1016/0303-7207(90)90211-p. [DOI] [PubMed] [Google Scholar]
- Waterfield M. D., Mayes E. L., Stroobant P., Bennet P. L., Young S., Goodfellow P. N., Banting G. S., Ozanne B. A monoclonal antibody to the human epidermal growth factor receptor. J Cell Biochem. 1982;20(2):149–161. doi: 10.1002/jcb.240200207. [DOI] [PubMed] [Google Scholar]
- Weber W., Gill G. N., Spiess J. Production of an epidermal growth factor receptor-related protein. Science. 1984 Apr 20;224(4646):294–297. doi: 10.1126/science.6324343. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Kyo E., Tsujino T., Sano T., Niimoto M., Tahara E. Expression of epidermal growth factor, transforming growth factor-alpha and their receptor genes in human gastric carcinomas; implication for autocrine growth. Jpn J Cancer Res. 1990 Jan;81(1):43–51. doi: 10.1111/j.1349-7006.1990.tb02505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



