Abstract
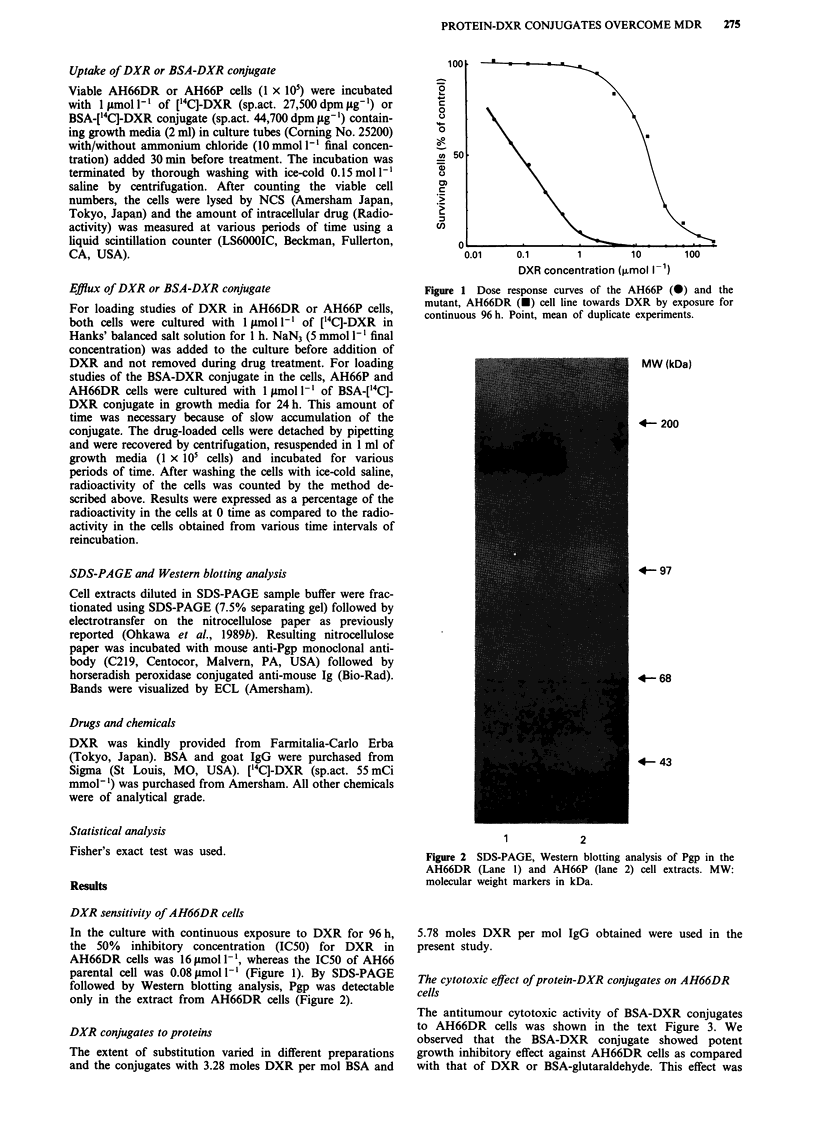
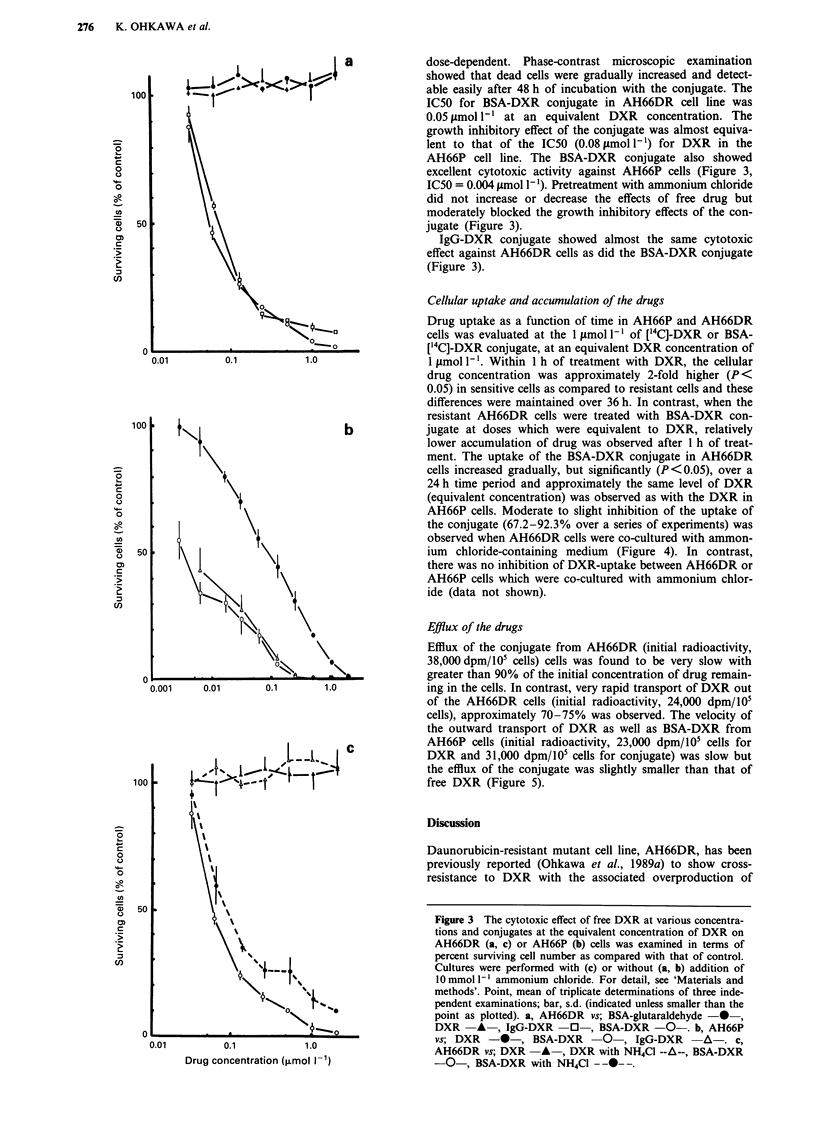
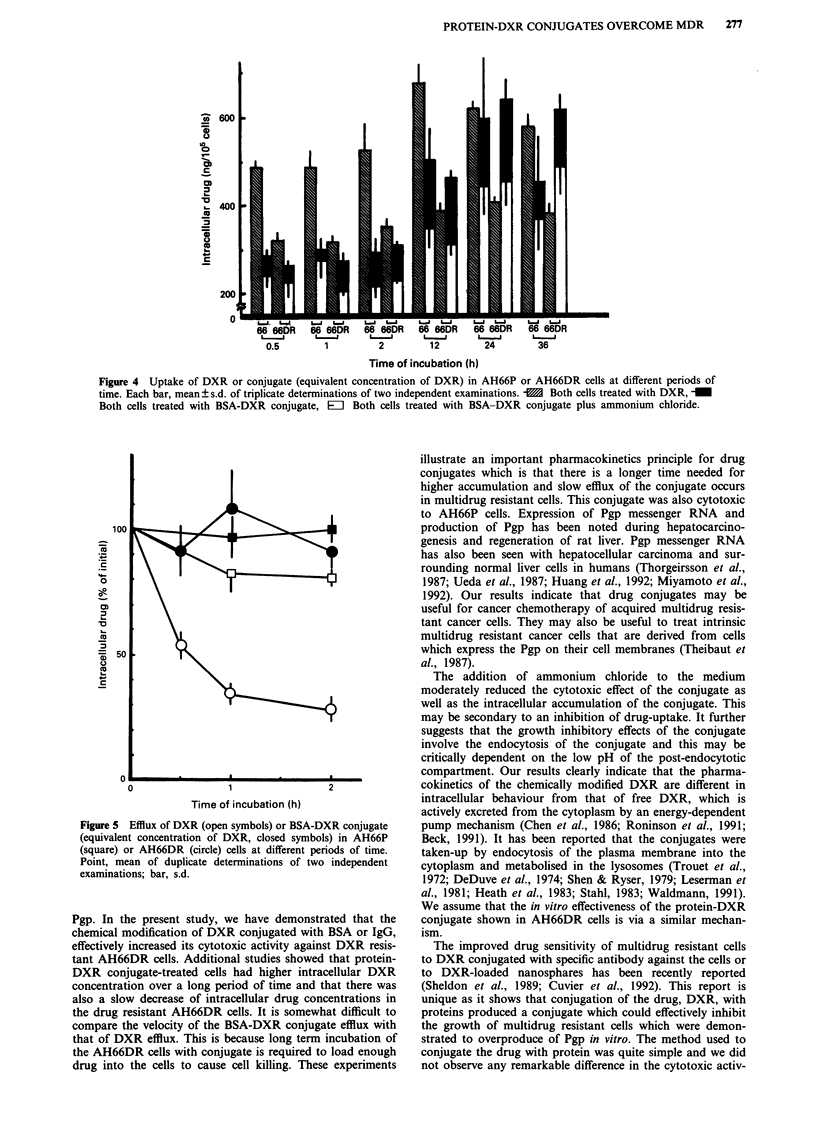
In vitro studies were initiated to study the antitumour effect of protein-doxorubicin (DXR) conjugate on the growth of the multidrug resistant rat ascites hepatoma cell line, AH66DR. The 50% inhibitory concentration (IC50) for DXR in AH66DR cell line was 16 mumol l-1 (AH66 parental cell line, AH66P, IC50 was 0.08 mumol l-1). Treatment of AH66P and AH66DR cells with various concentrations of DXR or conjugates at equivalent concentrations of DXR was performed. The two types of conjugates used were bovine serum albumin (BSA)-DXR conjugate and immunoglobulin G (IgG)-DXR conjugate. Both of these conjugates showed potent dose-dependent inhibition of cell growth against AH66DR cells as compared with the cells treated with DXR or other controls. The IC50 for BSA-DXR and IgG-DXR conjugates in AH66DR cell line was 0.05 (equivalent DXR) mumol l-1 and 0.07 (equivalent DXR) mumol l-1, respectively. These values were similar to that of the AH66P treated with DXR. Cellular uptake and accumulation of DXR or BSA-DXR conjugate was also quantitated in both cell lines. The cellular concentration of DXR in AH66DR cells was 2-fold lower than that of AH66P cells throughout the experiment. In contrast, by the treatment of AH66DR cells with BSA-DXR conjugate, the intracellular drug concentration increased as a function of time up to 24 h (639.1 +/- 41.8, equivalent DXR, ng 10(-5) cells) and reached the same drug level as AH66P cells treated with DXR (617.9 +/- 17.3 ng-5 cells). Ammonium chloride treatment inhibited the effects of the conjugates but did not inhibit the free drugs. Intracellular DXR was effluxed rapidly from AH66DR cells, but BSA-DXR conjugate remained in the cells at relatively high concentration for a long time. These results indicate that by chemically modifying DXR, such as by conjugation of the drug with proteins, it may be possible to overcome multidrug resistance.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck W. T. Do anti-P-glycoprotein antibodies have a future in the circumvention of multidrug resistance? J Natl Cancer Inst. 1991 Oct 2;83(19):1364–1366. doi: 10.1093/jnci/83.19.1364. [DOI] [PubMed] [Google Scholar]
- Chen A. Y., Yu C., Potmesil M., Wall M. E., Wani M. C., Liu L. F. Camptothecin overcomes MDR1-mediated resistance in human KB carcinoma cells. Cancer Res. 1991 Nov 15;51(22):6039–6044. [PubMed] [Google Scholar]
- Chen C. J., Chin J. E., Ueda K., Clark D. P., Pastan I., Gottesman M. M., Roninson I. B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986 Nov 7;47(3):381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- Coley H. M., Twentyman P. R., Workman P. 9-Alkyl, morpholinyl anthracyclines in the circumvention of multidrug resistance. Eur J Cancer. 1990;26(6):665–667. doi: 10.1016/0277-5379(90)90112-7. [DOI] [PubMed] [Google Scholar]
- Cuvier C., Roblot-Treupel L., Millot J. M., Lizard G., Chevillard S., Manfait M., Couvreur P., Poupon M. F. Doxorubicin-loaded nanospheres bypass tumor cell multidrug resistance. Biochem Pharmacol. 1992 Aug 4;44(3):509–517. doi: 10.1016/0006-2952(92)90443-m. [DOI] [PubMed] [Google Scholar]
- FitzGerald D. J., Willingham M. C., Cardarelli C. O., Hamada H., Tsuruo T., Gottesman M. M., Pastan I. A monoclonal antibody-Pseudomonas toxin conjugate that specifically kills multidrug-resistant cells. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4288–4292. doi: 10.1073/pnas.84.12.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath T. D., Montgomery J. A., Piper J. R., Papahadjopoulos D. Antibody-targeted liposomes: increase in specific toxicity of methotrexate-gamma-aspartate. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1377–1381. doi: 10.1073/pnas.80.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X. F., Martin T. J., Bell D. R., de Luise M., Zalcberg J. R. Combined use of cyclosporin A and verapamil in modulating multidrug resistance in human leukemia cell lines. Cancer Res. 1990 May 15;50(10):2953–2957. [PubMed] [Google Scholar]
- Huang C. C., Wu M. C., Xu G. W., Li D. Z., Cheng H., Tu Z. X., Jiang H. Q., Gu J. R. Overexpression of the MDR1 gene and P-glycoprotein in human hepatocellular carcinoma. J Natl Cancer Inst. 1992 Feb 19;84(4):262–264. doi: 10.1093/jnci/84.4.262. [DOI] [PubMed] [Google Scholar]
- Hurwitz E., Levy R., Maron R., Wilchek M., Arnon R., Sela M. The covalent binding of daunomycin and adriamycin to antibodies, with retention of both drug and antibody activities. Cancer Res. 1975 May;35(5):1175–1181. [PubMed] [Google Scholar]
- Kato Y., Tsukada Y., Hara T., Hirai H. Enhanced antitumor activity of mitomycin C conjugated with anti-alpha-fetoprotein antibody by a novel method of conjugation. J Appl Biochem. 1983 Aug-Oct;5(4-5):313–319. [PubMed] [Google Scholar]
- Leserman L. D., Machy P., Barbet J. Cell-specific drug transfer from liposomes bearing monoclonal antibodies. Nature. 1981 Sep 17;293(5829):226–228. doi: 10.1038/293226a0. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Wakusawa S., Nakamura S. Drug resistance dependent on different molecular size P-glycoproteins in Yoshida rat ascites hepatoma cells. Biochem Pharmacol. 1992 Mar 3;43(5):1143–1145. doi: 10.1016/0006-2952(92)90623-q. [DOI] [PubMed] [Google Scholar]
- Ohkawa K., Tsukada Y., Abe T., Takada K., Hibi N. Overcoming effect of antibody against rat alpha-fetoprotein (AFP) on the growth of daunorubicin-resistant mutant rat ascites hepatoma cell line AH66. Int J Cancer. 1989 Sep 15;44(3):489–493. doi: 10.1002/ijc.2910440319. [DOI] [PubMed] [Google Scholar]
- Ohkawa K., Tsukada Y., Hibi N., Umemoto N., Hara T. Selective in vitro and in vivo growth inhibition against human yolk sac tumor cell lines by purified antibody against human alpha-fetoprotein conjugated with mitomycin C via human serum albumin. Cancer Immunol Immunother. 1986;23(2):81–86. doi: 10.1007/BF00199811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa K., Tsukada Y., Murae M., Kimura E., Takada K., Abe T., Terashima Y., Mitani K. Serum levels and biochemical characteristics of human ovarian carcinoma-associated antigen defined by murine monoclonal antibody, CF511. Br J Cancer. 1989 Dec;60(6):953–960. doi: 10.1038/bjc.1989.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J. W., Fogler W. E., Volker K., Usui N., Goldenberg S. K., Gruys E., Riggs C. W., Komschlies K., Wiltrout R. H., Tsuruo T. Reversal of drug resistance in a human colon cancer xenograft expressing MDR1 complementary DNA by in vivo administration of MRK-16 monoclonal antibody. J Natl Cancer Inst. 1991 Oct 2;83(19):1386–1391. doi: 10.1093/jnci/83.19.1386. [DOI] [PubMed] [Google Scholar]
- Ripamonti M., Pezzoni G., Pesenti E., Pastori A., Farao M., Bargiotti A., Suarato A., Spreafico F., Grandi M. In vivo anti-tumour activity of FCE 23762, a methoxymorpholinyl derivative of doxorubicin active on doxorubicin-resistant tumour cells. Br J Cancer. 1992 May;65(5):703–707. doi: 10.1038/bjc.1992.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon K., Marks A., Baumal R. Sensitivity of multidrug resistant KB-C1 cells to an antibody-dextran-adriamycin conjugate. Anticancer Res. 1989 May-Jun;9(3):637–641. [PubMed] [Google Scholar]
- Shen W. C., Ryser H. J. Poly (L-lysine) and poly (D-lysine) conjugates of methotrexate: different inhibitory effect on drug resistant cells. Mol Pharmacol. 1979 Sep;16(2):614–622. [PubMed] [Google Scholar]
- Thiebaut F., Tsuruo T., Hamada H., Gottesman M. M., Pastan I., Willingham M. C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson S. S., Huber B. E., Sorrell S., Fojo A., Pastan I., Gottesman M. M. Expression of the multidrug-resistant gene in hepatocarcinogenesis and regenerating rat liver. Science. 1987 May 29;236(4805):1120–1122. doi: 10.1126/science.3576227. [DOI] [PubMed] [Google Scholar]
- Trouet A., Deprez-de Campeneere D., De Duve C. Chemotherapy through lysosomes with a DNA-daunorubicin complex. Nat New Biol. 1972 Sep 27;239(91):110–112. doi: 10.1038/newbio239110a0. [DOI] [PubMed] [Google Scholar]
- Tsukada Y., Bischof W. K., Hibi N., Hirai H., Hurwitz E., Sela M. Effect of a conjugate of daunomycin and antibodies to rat alpha-fetoprotein on the growth of alpha-fetoprotein-producing tumor cells. Proc Natl Acad Sci U S A. 1982 Jan;79(2):621–625. doi: 10.1073/pnas.79.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada Y., Kato Y., Umemoto N., Takeda Y., Hara T., Hirai H. An anti-alpha-fetoprotein antibody-daunorubicin conjugate with a novel poly-L-glutamic acid derivative as intermediate drug carrier. J Natl Cancer Inst. 1984 Sep;73(3):721–729. [PubMed] [Google Scholar]
- Tsuruo T., Hamada H., Sato S., Heike Y. Inhibition of multidrug-resistant human tumor growth in athymic mice by anti-P-glycoprotein monoclonal antibodies. Jpn J Cancer Res. 1989 Jul;80(7):627–631. doi: 10.1111/j.1349-7006.1989.tb01688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Increased accumulation of vincristine and adriamycin in drug-resistant P388 tumor cells following incubation with calcium antagonists and calmodulin inhibitors. Cancer Res. 1982 Nov;42(11):4730–4733. [PubMed] [Google Scholar]
- Twentyman P. R., Fox N. E., White D. J. Cyclosporin A and its analogues as modifiers of adriamycin and vincristine resistance in a multi-drug resistant human lung cancer cell line. Br J Cancer. 1987 Jul;56(1):55–57. doi: 10.1038/bjc.1987.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K., Clark D. P., Chen C. J., Roninson I. B., Gottesman M. M., Pastan I. The human multidrug resistance (mdr1) gene. cDNA cloning and transcription initiation. J Biol Chem. 1987 Jan 15;262(2):505–508. [PubMed] [Google Scholar]
- Waldmann T. A. Monoclonal antibodies in diagnosis and therapy. Science. 1991 Jun 21;252(5013):1657–1662. doi: 10.1126/science.2047874. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Komeshima N., Nakajima S., Tsuruo T. MX2, a morpholino anthracycline, as a new antitumor agent against drug-sensitive and multidrug-resistant human and murine tumor cells. Cancer Res. 1988 Dec 1;48(23):6653–6657. [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]



