Abstract
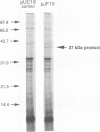
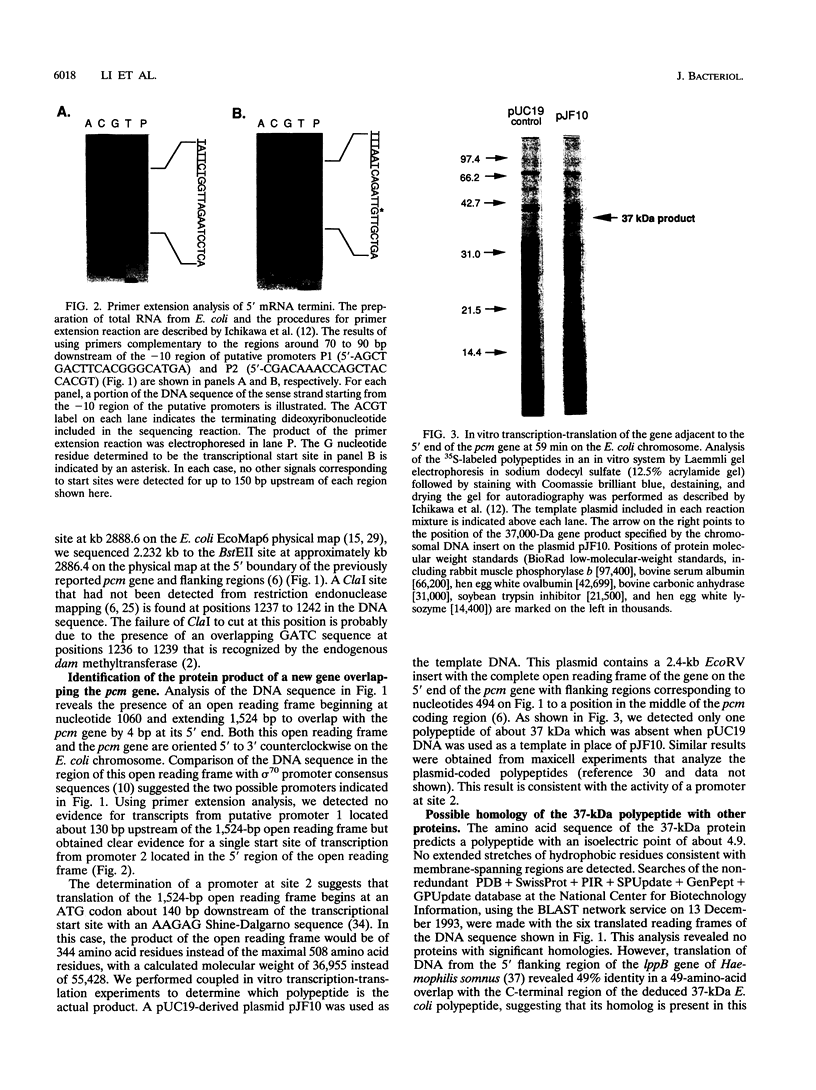
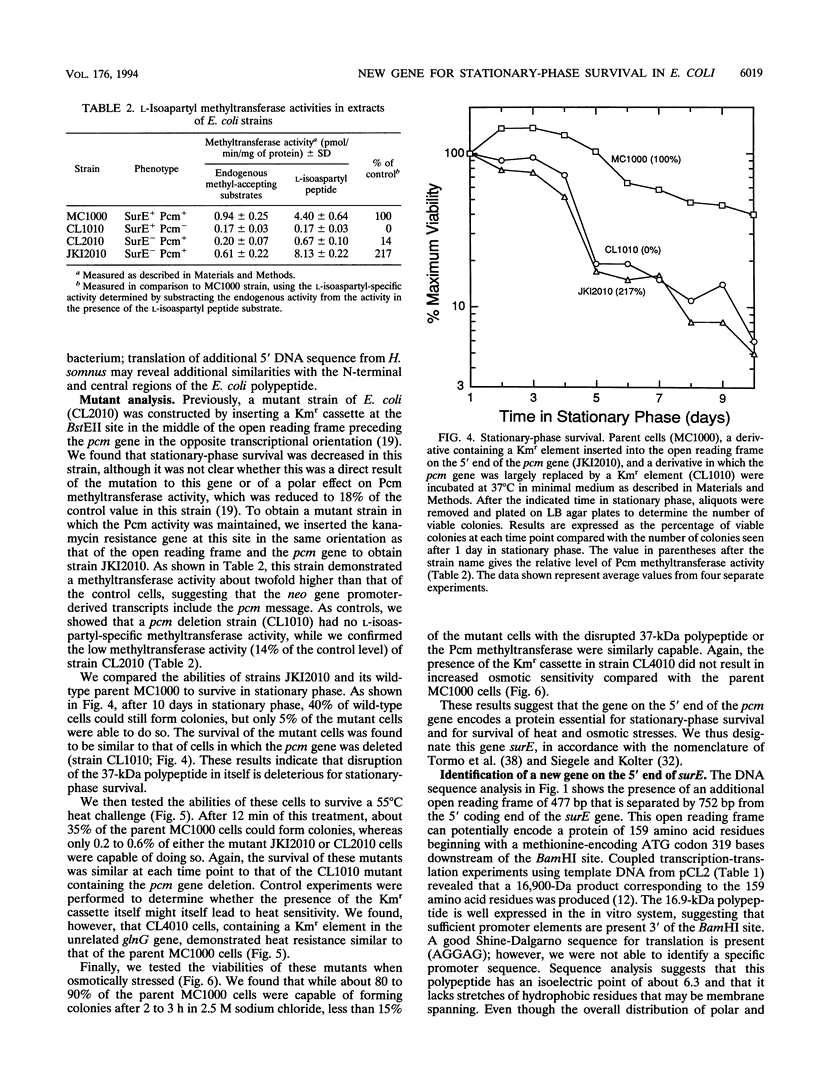
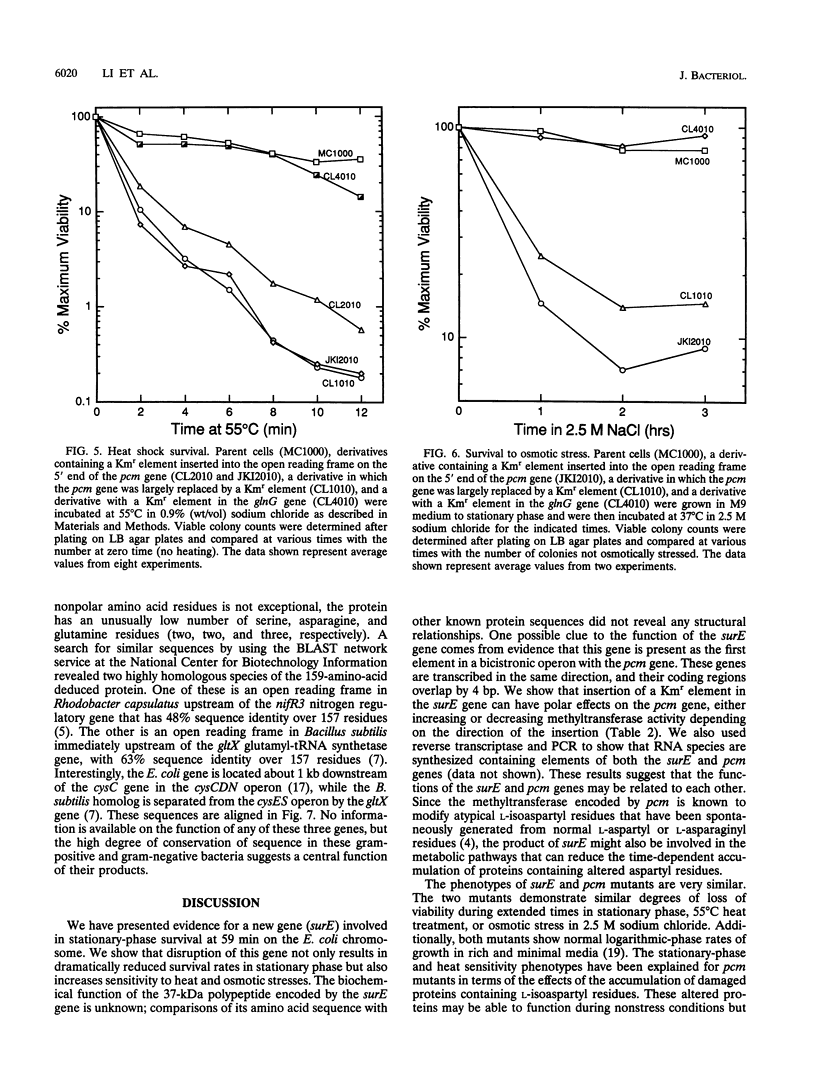
We determined the DNA sequence of a 2,232-bp region immediately upstream of the pcm gene at 59 min on the Escherichia coli chromosome that encodes an L-isoaspartyl protein methyltransferase with an important role in stationary-phase survival. Two open reading frames of 477 and 1,524 bp were found oriented in the same direction as that of the pcm gene. The latter open reading frame overlapped the 5' end of the pcm gene by 4 bp. Coupled in vitro transcription-translation analysis of DNA containing the 1,524-bp open reading frame directly demonstrated the production of a 37,000-Da polypeptide corresponding to a RNA species generated from a promoter within the open reading frame. The deduced amino acid sequence showed no similarity to known protein sequences. To test the function of this gene product, we constructed a mutant strain in which a kanamycin resistance element was inserted at a BstEII site in the middle of its coding region in an orientation that does not result in reduction of Pcm methyltransferase activity. These cells were found to survive poorly in stationary phase, at elevated temperatures, and in high-salt media compared with parent cells containing the intact gene, and we thus designate this gene surE (survival). surE appears to be the first gene of a bicistronic operon also containing the pcm gene. The phenotypes of mutations in either gene are very similar and indicate that both gene products are important for the viability of E. coli cells under stressful conditions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Casjens S., Hayden M., Jackson E., Deans R. Additional restriction endonuclease cleavage sites on the bacteriophage P22 genome. J Virol. 1983 Feb;45(2):864–867. doi: 10.1128/jvi.45.2.864-867.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. T., Niemela S. L., Miller R. H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S. Protein carboxyl methyltransferases: two distinct classes of enzymes. Annu Rev Biochem. 1985;54:479–506. doi: 10.1146/annurev.bi.54.070185.002403. [DOI] [PubMed] [Google Scholar]
- Foster-Hartnett D., Cullen P. J., Gabbert K. K., Kranz R. G. Sequence, genetic, and lacZ fusion analyses of a nifR3-ntrB-ntrC operon in Rhodobacter capsulatus. Mol Microbiol. 1993 May;8(5):903–914. doi: 10.1111/j.1365-2958.1993.tb01636.x. [DOI] [PubMed] [Google Scholar]
- Fu J. C., Ding L., Clarke S. Purification, gene cloning, and sequence analysis of an L-isoaspartyl protein carboxyl methyltransferase from Escherichia coli. J Biol Chem. 1991 Aug 5;266(22):14562–14572. [PubMed] [Google Scholar]
- Gagnon Y., Breton R., Putzer H., Pelchat M., Grunberg-Manago M., Lapointe J. Clustering and co-transcription of the Bacillus subtilis genes encoding the aminoacyl-tRNA synthetases specific for glutamate and for cysteine and the first enzyme for cysteine biosynthesis. J Biol Chem. 1994 Mar 11;269(10):7473–7482. [PubMed] [Google Scholar]
- Haley E. E. Purification and properties of a beta-aspartyl peptidase from Escherichia coli. J Biol Chem. 1968 Nov 10;243(21):5748–5752. [PubMed] [Google Scholar]
- Harley C. B., Reynolds R. P. Analysis of E. coli promoter sequences. Nucleic Acids Res. 1987 Mar 11;15(5):2343–2361. doi: 10.1093/nar/15.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengge-Aronis R. Survival of hunger and stress: the role of rpoS in early stationary phase gene regulation in E. coli. Cell. 1993 Jan 29;72(2):165–168. doi: 10.1016/0092-8674(93)90655-a. [DOI] [PubMed] [Google Scholar]
- Ichikawa J. K., Li C., Fu J., Clarke S. A gene at 59 minutes on the Escherichia coli chromosome encodes a lipoprotein with unusual amino acid repeat sequences. J Bacteriol. 1994 Mar;176(6):1630–1638. doi: 10.1128/jb.176.6.1630-1638.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. A., Murray E. D., Jr, Clarke S., Glass D. B., Aswad D. W. Protein carboxyl methyltransferase facilitates conversion of atypical L-isoaspartyl peptides to normal L-aspartyl peptides. J Biol Chem. 1987 Apr 25;262(12):5622–5629. [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Kolter R., Siegele D. A., Tormo A. The stationary phase of the bacterial life cycle. Annu Rev Microbiol. 1993;47:855–874. doi: 10.1146/annurev.mi.47.100193.004231. [DOI] [PubMed] [Google Scholar]
- Leyh T. S., Taylor J. C., Markham G. D. The sulfate activation locus of Escherichia coli K12: cloning, genetic, and enzymatic characterization. J Biol Chem. 1988 Feb 15;263(5):2409–2416. [PubMed] [Google Scholar]
- Li C., Clarke S. A protein methyltransferase specific for altered aspartyl residues is important in Escherichia coli stationary-phase survival and heat-shock resistance. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9885–9889. doi: 10.1073/pnas.89.20.9885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Clarke S. Distribution of an L-isoaspartyl protein methyltransferase in eubacteria. J Bacteriol. 1992 Jan;174(2):355–361. doi: 10.1128/jb.174.2.355-361.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Auger E. A., Blum P. H., Schultz J. E. Genetic basis of starvation survival in nondifferentiating bacteria. Annu Rev Microbiol. 1989;43:293–316. doi: 10.1146/annurev.mi.43.100189.001453. [DOI] [PubMed] [Google Scholar]
- McCann M. P., Fraley C. D., Matin A. The putative sigma factor KatF is regulated posttranscriptionally during carbon starvation. J Bacteriol. 1993 Apr;175(7):2143–2149. doi: 10.1128/jb.175.7.2143-2149.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden P. N., Clarke S. Conversion of isoaspartyl peptides to normal peptides: implications for the cellular repair of damaged proteins. Proc Natl Acad Sci U S A. 1987 May;84(9):2595–2599. doi: 10.1073/pnas.84.9.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden P. N., Clarke S. Methylation at D-aspartyl residues in erythrocytes: possible step in the repair of aged membrane proteins. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2460–2464. doi: 10.1073/pnas.79.8.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvey M. R., Sorby P. A., Triggs-Raine B. L., Loewen P. C. Cloning and physical characterization of katE and katF required for catalase HPII expression in Escherichia coli. Gene. 1988 Dec 20;73(2):337–345. doi: 10.1016/0378-1119(88)90498-2. [DOI] [PubMed] [Google Scholar]
- Nguyen L. H., Jensen D. B., Thompson N. E., Gentry D. R., Burgess R. R. In vitro functional characterization of overproduced Escherichia coli katF/rpoS gene product. Biochemistry. 1993 Oct 19;32(41):11112–11117. doi: 10.1021/bi00092a021. [DOI] [PubMed] [Google Scholar]
- O'Connor C. M., Clarke S. Specific recognition of altered polypeptides by widely distributed methyltransferases. Biochem Biophys Res Commun. 1985 Nov 15;132(3):1144–1150. doi: 10.1016/0006-291x(85)91926-6. [DOI] [PubMed] [Google Scholar]
- Roszak D. B., Colwell R. R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987 Sep;51(3):365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Wharton R. P., Seltzer S., Kacinski B. M., Clarke N. D., Rupp W. D. Identification of the uvrA gene product. J Mol Biol. 1981 May 5;148(1):45–62. doi: 10.1016/0022-2836(81)90234-5. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegele D. A., Kolter R. Isolation and characterization of an Escherichia coli mutant defective in resuming growth after starvation. Genes Dev. 1993 Dec;7(12B):2629–2640. doi: 10.1101/gad.7.12b.2629. [DOI] [PubMed] [Google Scholar]
- Siegele D. A., Kolter R. Life after log. J Bacteriol. 1992 Jan;174(2):345–348. doi: 10.1128/jb.174.2.345-348.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Takayanagi Y., Fujita N., Ishihama A., Takahashi H. Heterogeneity of the principal sigma factor in Escherichia coli: the rpoS gene product, sigma 38, is a second principal sigma factor of RNA polymerase in stationary-phase Escherichia coli. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3511–3515. doi: 10.1073/pnas.90.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen M., Rioux C. R., Potter A. A. Molecular cloning, nucleotide sequence, and characterization of lppB, encoding an antigenic 40-kilodalton lipoprotein of Haemophilus somnus. Infect Immun. 1993 May;61(5):1793–1798. doi: 10.1128/iai.61.5.1793-1798.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormo A., Almirón M., Kolter R. surA, an Escherichia coli gene essential for survival in stationary phase. J Bacteriol. 1990 Aug;172(8):4339–4347. doi: 10.1128/jb.172.8.4339-4347.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichart D., Lange R., Henneberg N., Hengge-Aronis R. Identification and characterization of stationary phase-inducible genes in Escherichia coli. Mol Microbiol. 1993 Oct;10(2):407–420. [PubMed] [Google Scholar]
- Winans S. C., Elledge S. J., Krueger J. H., Walker G. C. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1219–1221. doi: 10.1128/jb.161.3.1219-1221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]