Abstract
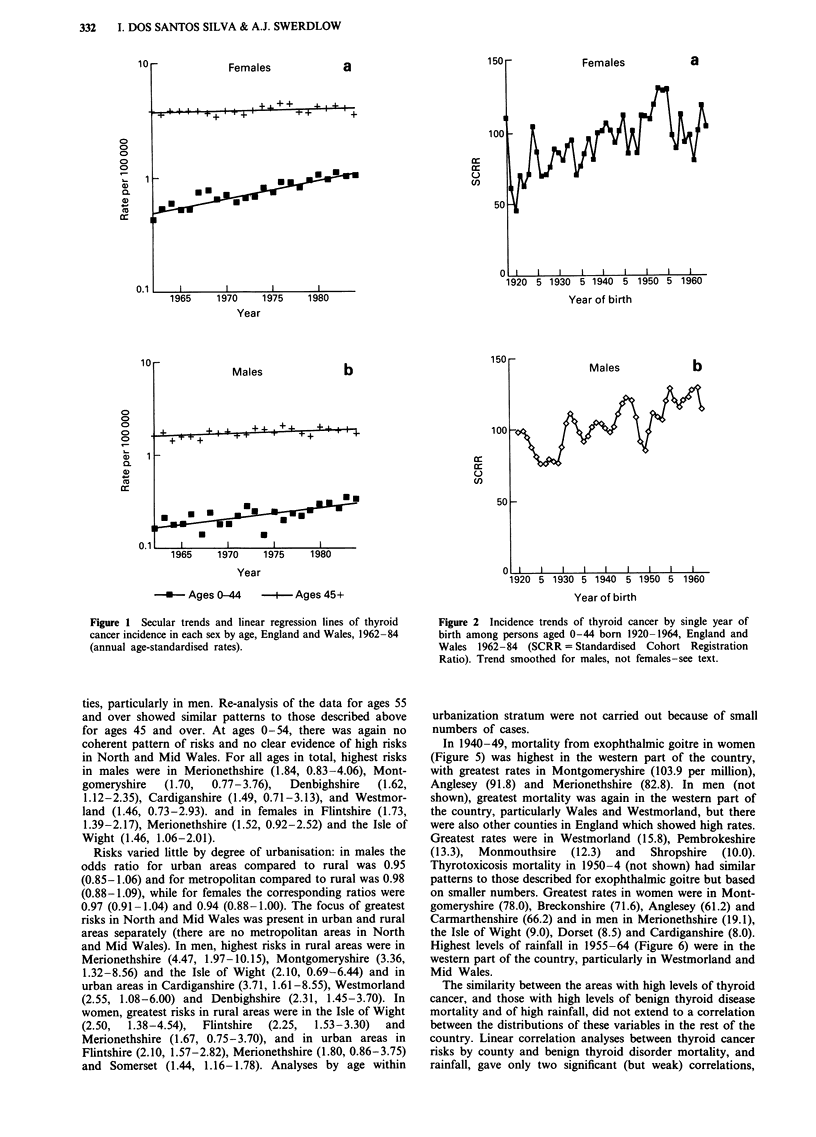
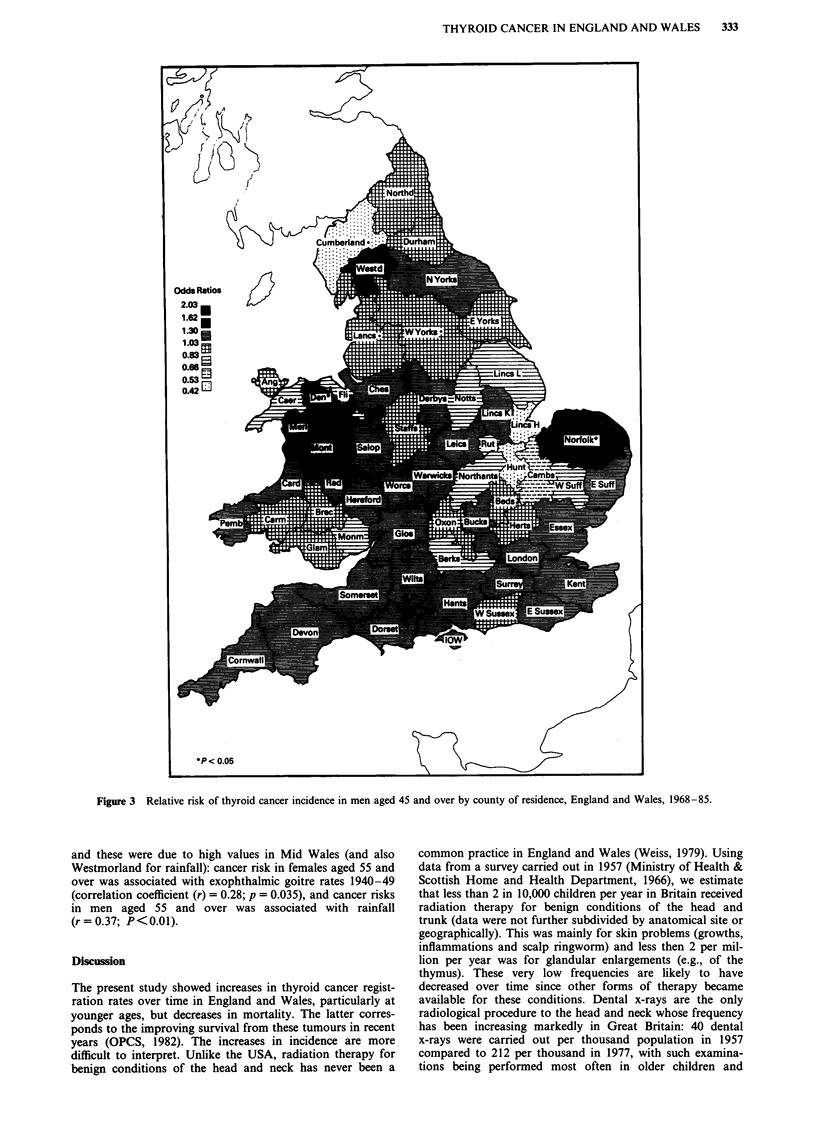
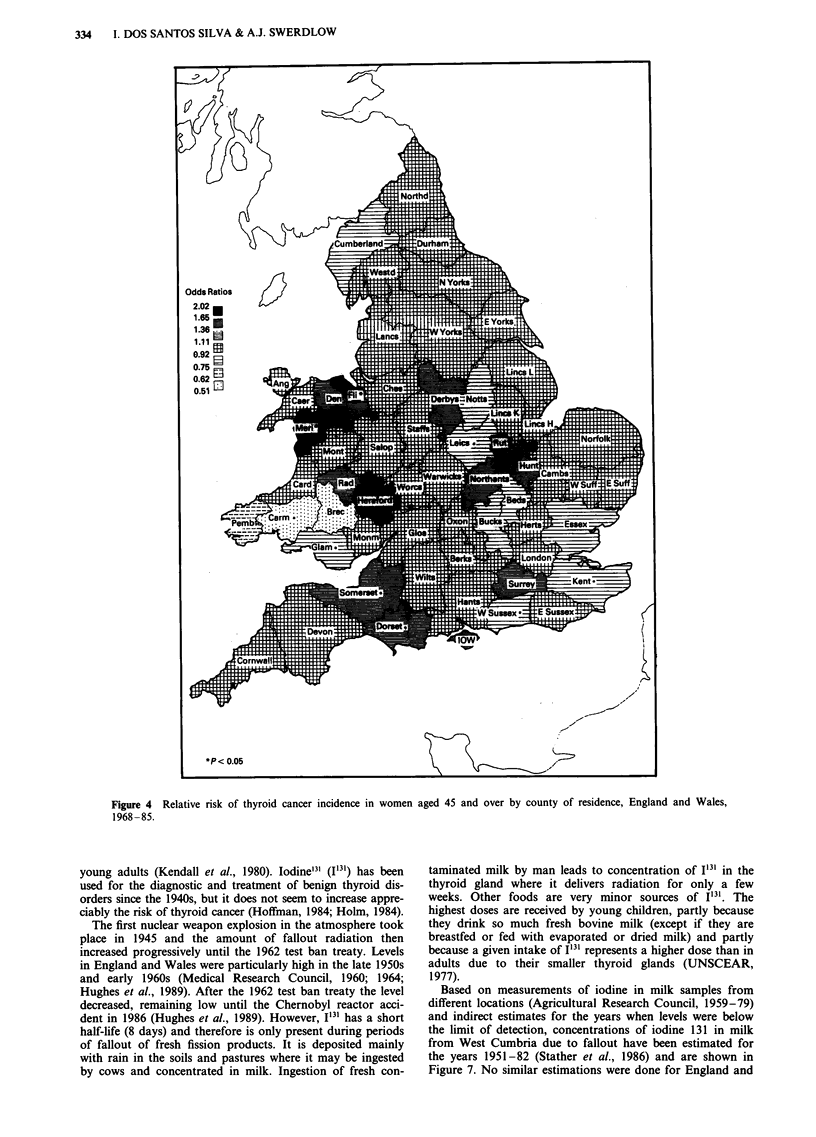
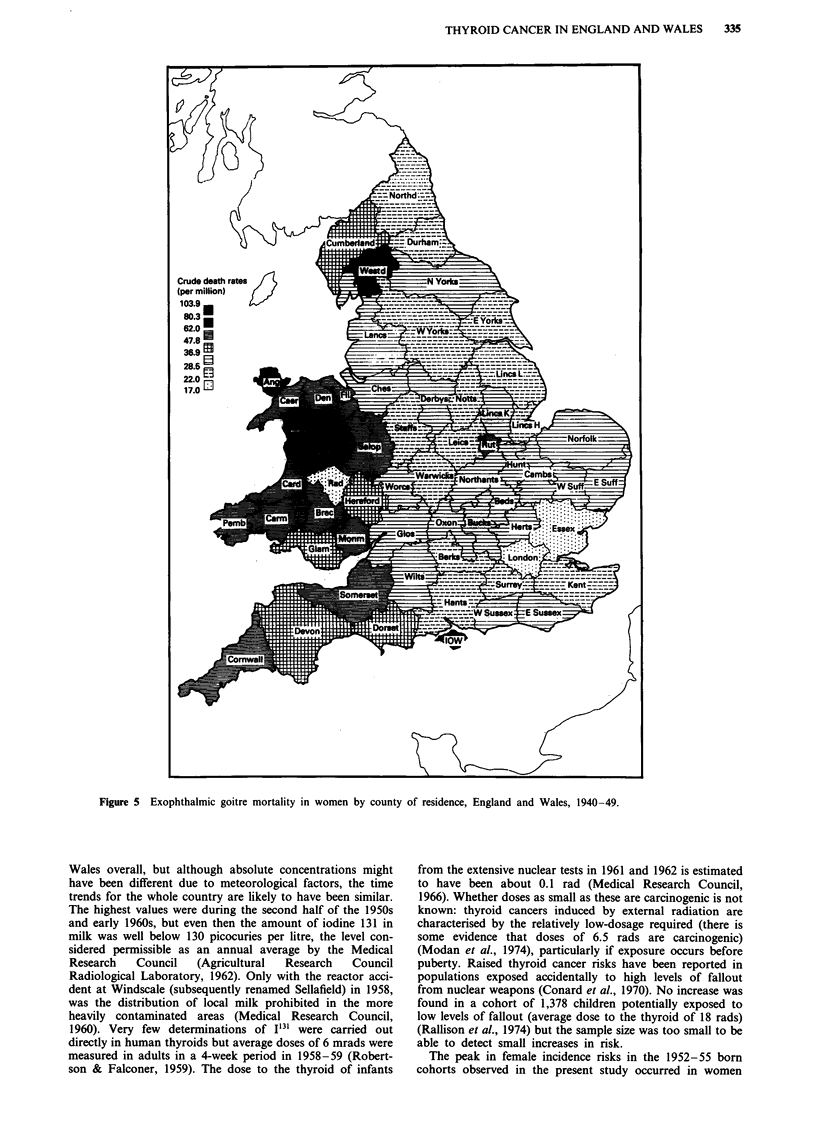
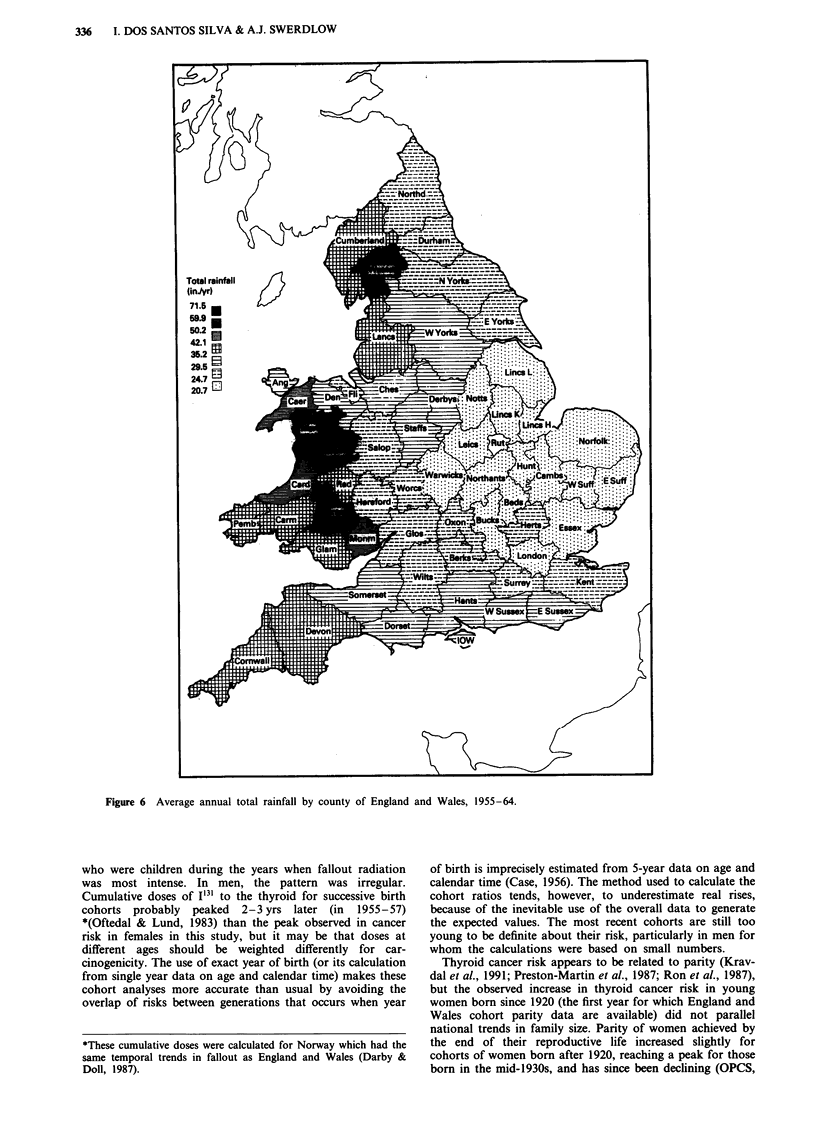
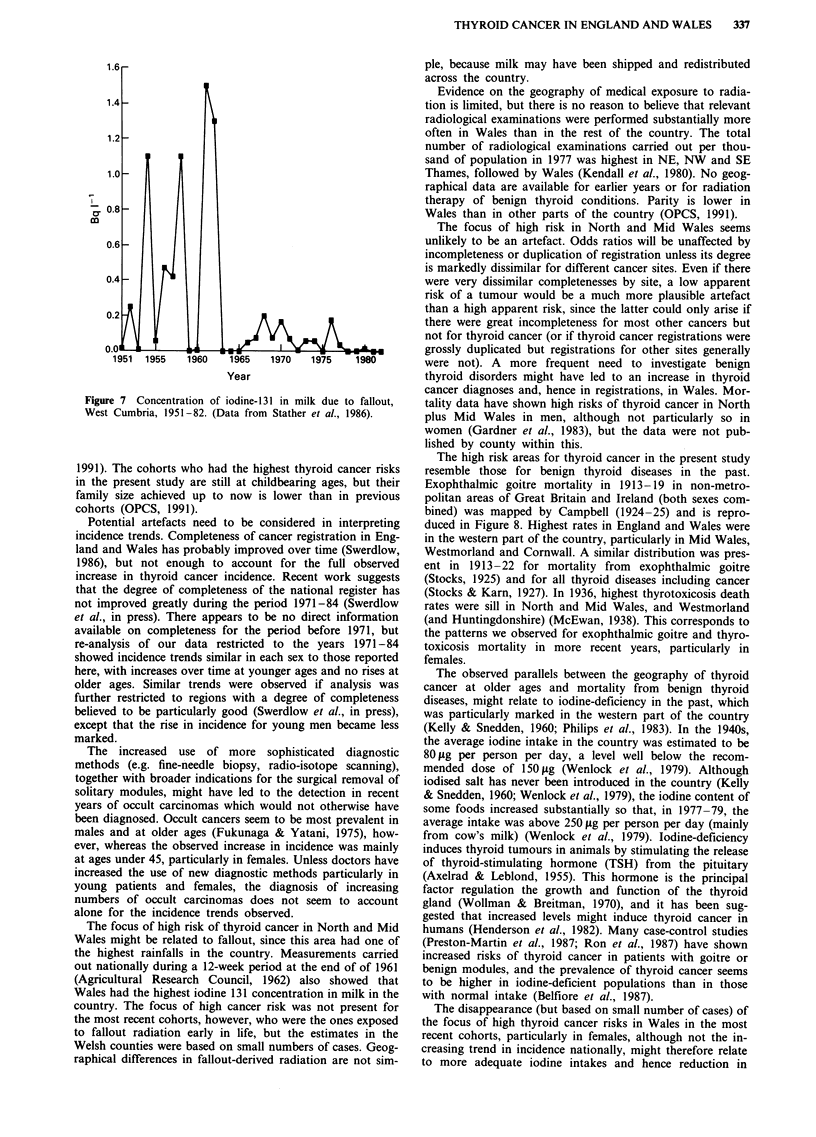
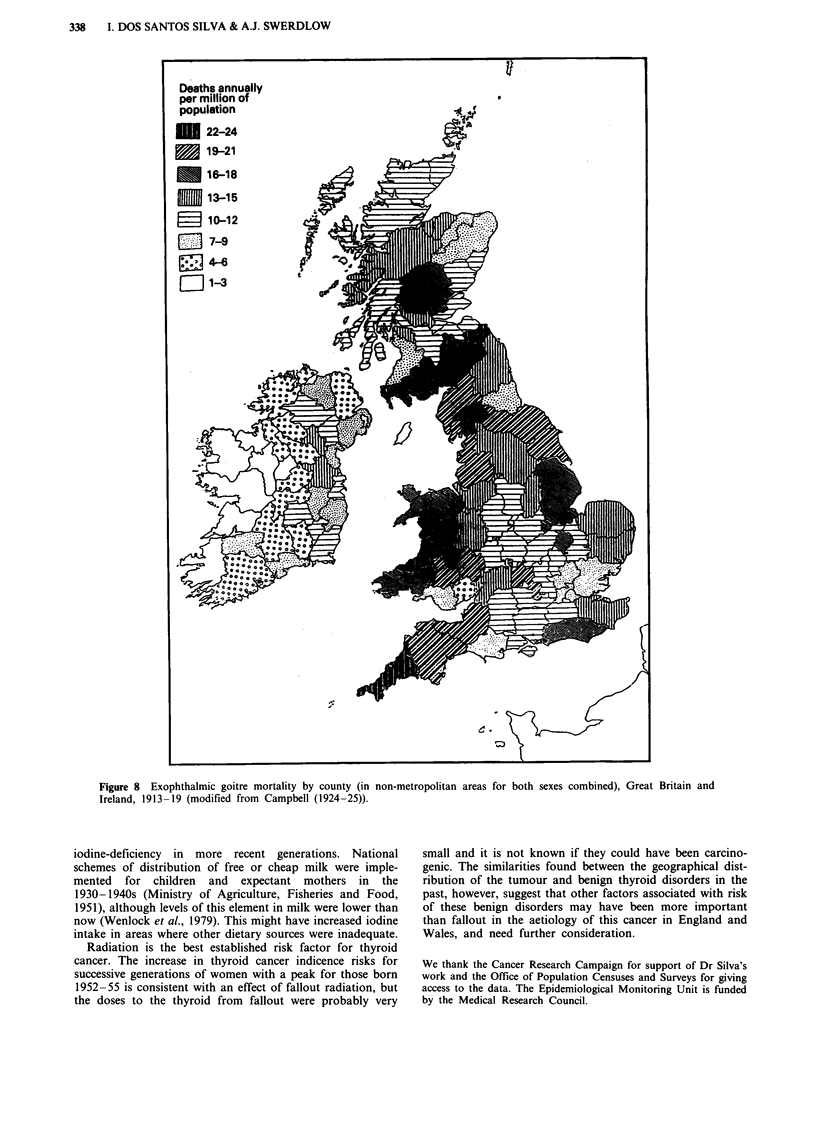
Thyroid cancer incidence has been increasing in many countries, whereas mortality has been falling due to better survival. Radiation is the best-established risk factor and there has been concern that recent rises in incidence might be related to fallout radiation from atmospheric nuclear weapon tests. We examined thyroid cancer time trends and geographical distribution in England and Wales and possible interpretations of these. During 1962-84, there were significant increases in incidence (P < 0.001) in each sex at ages under 45. Cohort analysis by single year of birth showed an overall increase in incidence risks in women aged 0-44 born since 1920, with a sudden rise in risk for the birth years 1952-55 followed by a lower risk for the more recent cohorts. In men, there was an overall increase in risk at ages 0-44 in successive birth cohorts, but the pattern was irregular. In each sex, the risk in persons aged 45 and over decreased slightly in successive generations. Geographically, highest incidence risks were in countries in North and Mid Wales, in which the risk was almost twice that in the rest of the country. This pattern was present only at ages 45 and over and was most clear in rural areas. The peak of thyroid cancer risk in women born in 1952-55 is consistent with a carcinogenic effect of fallout radiation, since these women were children in the late 1950s and early 1960s when fallout radiation was greatest in England and Wales. The focus of high thyroid cancer risks in Wales was in areas with high levels of fallout radiation. However, thyroid cancer risks in Wales were not high for more recent cohorts (the ones who were exposed to fallout early in life), and a focus on high risk of benign thyroid diseases was present in Wales well before nuclear weapons existed. The distributions of these benign thyroid diseases, or of factors causing them, seem more likely than fallout to explain the high risk areas for thyroid cancer in the country.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELRAD A. A., LEBLOND C. P. Induction of thyroid tumors in rats by a low iodine diet. Cancer. 1955 Mar-Apr;8(2):339–367. doi: 10.1002/1097-0142(1955)8:2<339::aid-cncr2820080214>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Belfiore A., La Rosa G. L., Padova G., Sava L., Ippolito O., Vigneri R. The frequency of cold thyroid nodules and thyroid malignancies in patients from an iodine-deficient area. Cancer. 1987 Dec 15;60(12):3096–3102. doi: 10.1002/1097-0142(19871215)60:12<3096::aid-cncr2820601240>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Beral V., Fraser P., Chilvers C. Does pregnancy protect against ovarian cancer? Lancet. 1978 May 20;1(8073):1083–1087. doi: 10.1016/s0140-6736(78)90925-x. [DOI] [PubMed] [Google Scholar]
- CAMPBELL H., DOLL W. R., LETCHNER J. THE INCIDENCE OF THYROID CANCER IN ENGLAND AND WALES. Br Med J. 1963 Nov 30;2(5369):1370–1373. doi: 10.1136/bmj.2.5369.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conard R. A., Dobyns B. M., Sutow W. W. Thyroid neoplasia as late effect of exposure to radioactive iodine in fallout. JAMA. 1970 Oct 12;214(2):316–324. [PubMed] [Google Scholar]
- Darby S. C., Doll R. Fallout, radiation doses near Dounreay, and childhood leukaemia. Br Med J (Clin Res Ed) 1987 Mar 7;294(6572):603–607. doi: 10.1136/bmj.294.6572.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGroot L., Paloyan E. Thyroid carcinoma and radiation. A Chicago endemic. JAMA. 1973 Jul 30;225(5):487–491. [PubMed] [Google Scholar]
- Favus M. J., Schneider A. B., Stachura M. E., Arnold J. E., Ryo U. Y., Pinsky S. M., Colman M., Arnold M. J., Frohman L. A. Thyroid cancer occurring as a late consequence of head-and-neck irradiation. Evaluation of 1056 patients. N Engl J Med. 1976 May 6;294(19):1019–1025. doi: 10.1056/NEJM197605062941901. [DOI] [PubMed] [Google Scholar]
- Fukunaga F. H., Yatani R. Geographic pathology of occult thyroid carcinomas. Cancer. 1975 Sep;36(3):1095–1099. doi: 10.1002/1097-0142(197509)36:3<1095::aid-cncr2820360338>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Henderson B. E., Ross R. K., Pike M. C., Casagrande J. T. Endogenous hormones as a major factor in human cancer. Cancer Res. 1982 Aug;42(8):3232–3239. [PubMed] [Google Scholar]
- Kravdal O., Glattre E., Haldorsen T. Positive correlation between parity and incidence of thyroid cancer: new evidence based on complete Norwegian birth cohorts. Int J Cancer. 1991 Dec 2;49(6):831–836. doi: 10.1002/ijc.2910490606. [DOI] [PubMed] [Google Scholar]
- MANTEL N., HAENSZEL W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959 Apr;22(4):719–748. [PubMed] [Google Scholar]
- McTiernan A., Weiss N. S., Daling J. R. Incidence of thyroid cancer in women in relation to known or suspected risk factors for breast cancer. Cancer Res. 1987 Jan 1;47(1):292–295. [PubMed] [Google Scholar]
- Miettinen O. Estimability and estimation in case-referent studies. Am J Epidemiol. 1976 Feb;103(2):226–235. doi: 10.1093/oxfordjournals.aje.a112220. [DOI] [PubMed] [Google Scholar]
- Modan B., Baidatz D., Mart H., Steinitz R., Levin S. G. Radiation-induced head and neck tumours. Lancet. 1974 Feb 23;1(7852):277–279. doi: 10.1016/s0140-6736(74)92592-6. [DOI] [PubMed] [Google Scholar]
- Pettersson B., Adami H. O., Wilander E., Coleman M. P. Trends in thyroid cancer incidence in Sweden, 1958-1981, by histopathologic type. Int J Cancer. 1991 Apr 22;48(1):28–33. doi: 10.1002/ijc.2910480106. [DOI] [PubMed] [Google Scholar]
- Phillips D. I., Barker D. J., Winter P. D., Osmond C. Mortality from thyrotoxicosis in England and Wales and its association with the previous prevalence of endemic goitre. J Epidemiol Community Health. 1983 Dec;37(4):305–309. doi: 10.1136/jech.37.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottern L. M., Stone B. J., Day N. E., Pickle L. W., Fraumeni J. F., Jr Thyroid cancer in Connecticut, 1935-1975: an analysis by cell type. Am J Epidemiol. 1980 Dec;112(6):764–774. doi: 10.1093/oxfordjournals.aje.a113049. [DOI] [PubMed] [Google Scholar]
- Preston-Martin S., Bernstein L., Pike M. C., Maldonado A. A., Henderson B. E. Thyroid cancer among young women related to prior thyroid disease and pregnancy history. Br J Cancer. 1987 Feb;55(2):191–195. doi: 10.1038/bjc.1987.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTSON H. A., FALCONER I. R. Accumulation of radioactive iodine in thyroid glands subsequent to nuclear weapon tests and the accident at Windscale. Nature. 1959 Nov 28;184:1699–1702. doi: 10.1038/1841699a0. [DOI] [PubMed] [Google Scholar]
- Rallison M. L., Dobyns B. M., Keating F. R., Rall J. E., Tyler F. H. Thyroid disease in children. A survey of subjects potentially exposed to fallout radiation. Am J Med. 1974 Apr;56(4):457–463. doi: 10.1016/0002-9343(74)90476-8. [DOI] [PubMed] [Google Scholar]
- Ron E., Kleinerman R. A., Boice J. D., Jr, LiVolsi V. A., Flannery J. T., Fraumeni J. F., Jr A population-based case-control study of thyroid cancer. J Natl Cancer Inst. 1987 Jul;79(1):1–12. [PubMed] [Google Scholar]
- SOCOLOW E. L., HASHIZUME A., NERIISHI S., NIITANI R. Thyroid carcinoma in man after exposure to ionizing radiation. A summary of the findings in Hiroshima and Nagasaki. N Engl J Med. 1963 Feb 21;268:406–410. doi: 10.1056/NEJM196302212680803. [DOI] [PubMed] [Google Scholar]
- Weiss W. Changing incidence of thyroid cancer. J Natl Cancer Inst. 1979 May;62(5):1137–1142. [PubMed] [Google Scholar]
- Wenlock R. W., Buss D. H., Moxon R. E., Bunton N. G. Trace nutrients. 4. Iodine in British food. Br J Nutr. 1982 May;47(3):381–390. doi: 10.1079/bjn19820049. [DOI] [PubMed] [Google Scholar]
- Wollman S. H., Breitman T. R. Changes in DNA and weight of thyroid glands during hyperplasia and involution. Endocrinology. 1970 Feb;86(2):322–327. doi: 10.1210/endo-86-2-322. [DOI] [PubMed] [Google Scholar]


