Abstract
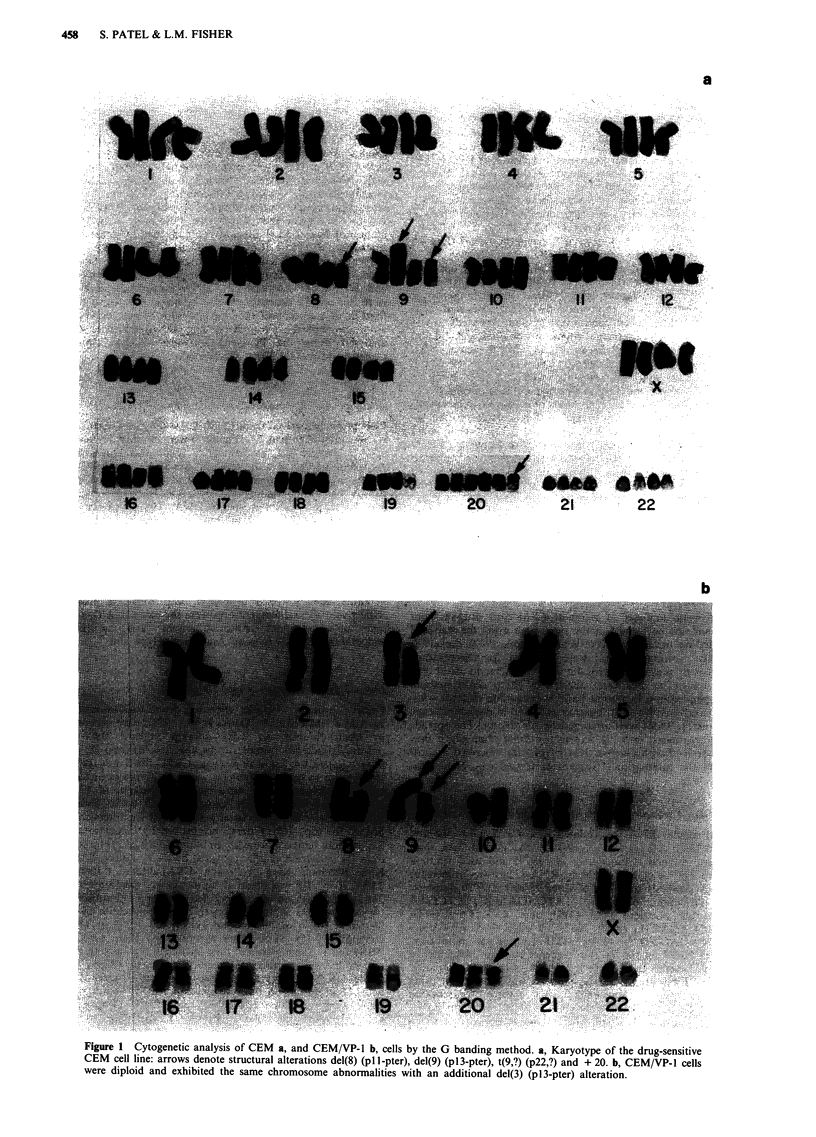
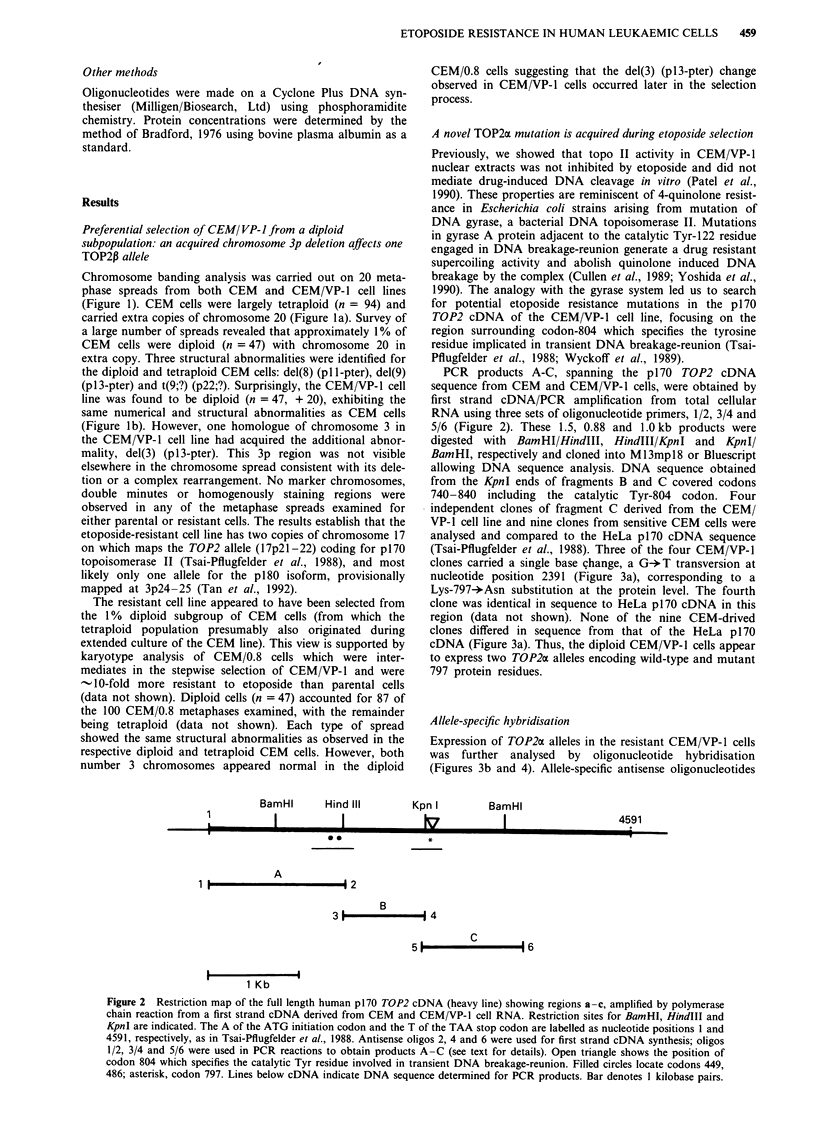
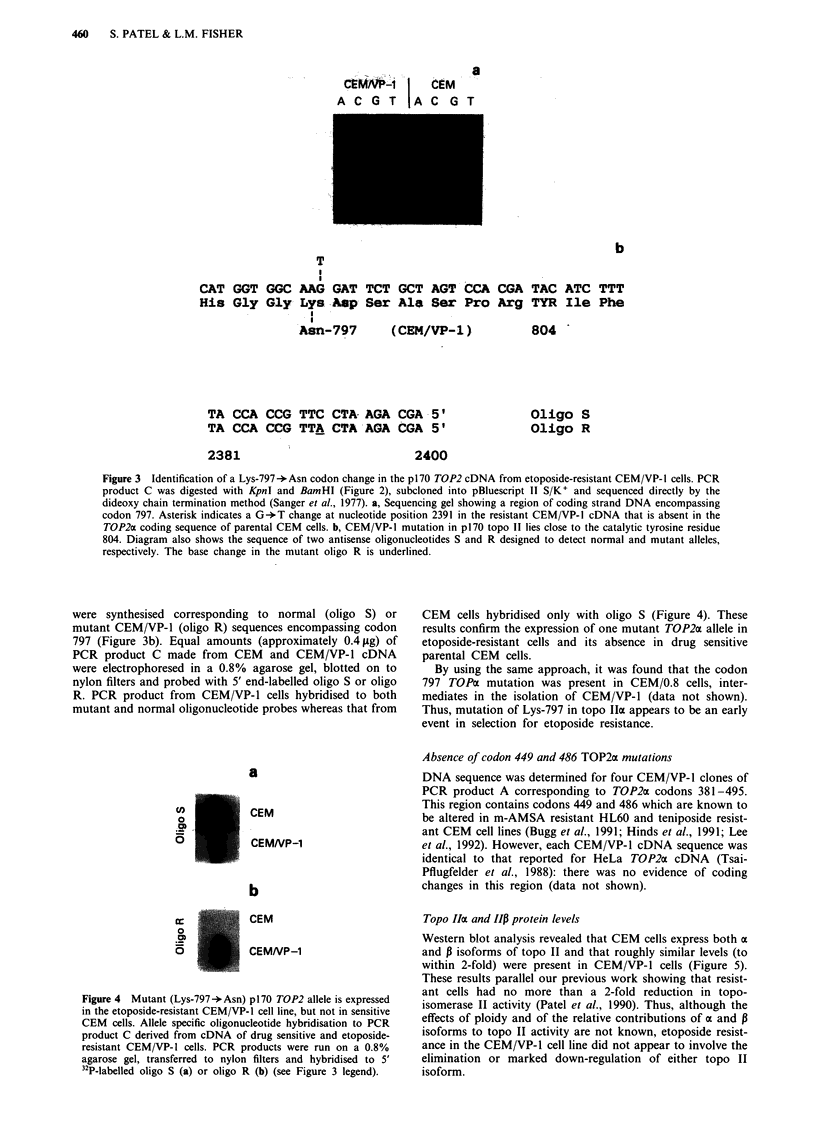
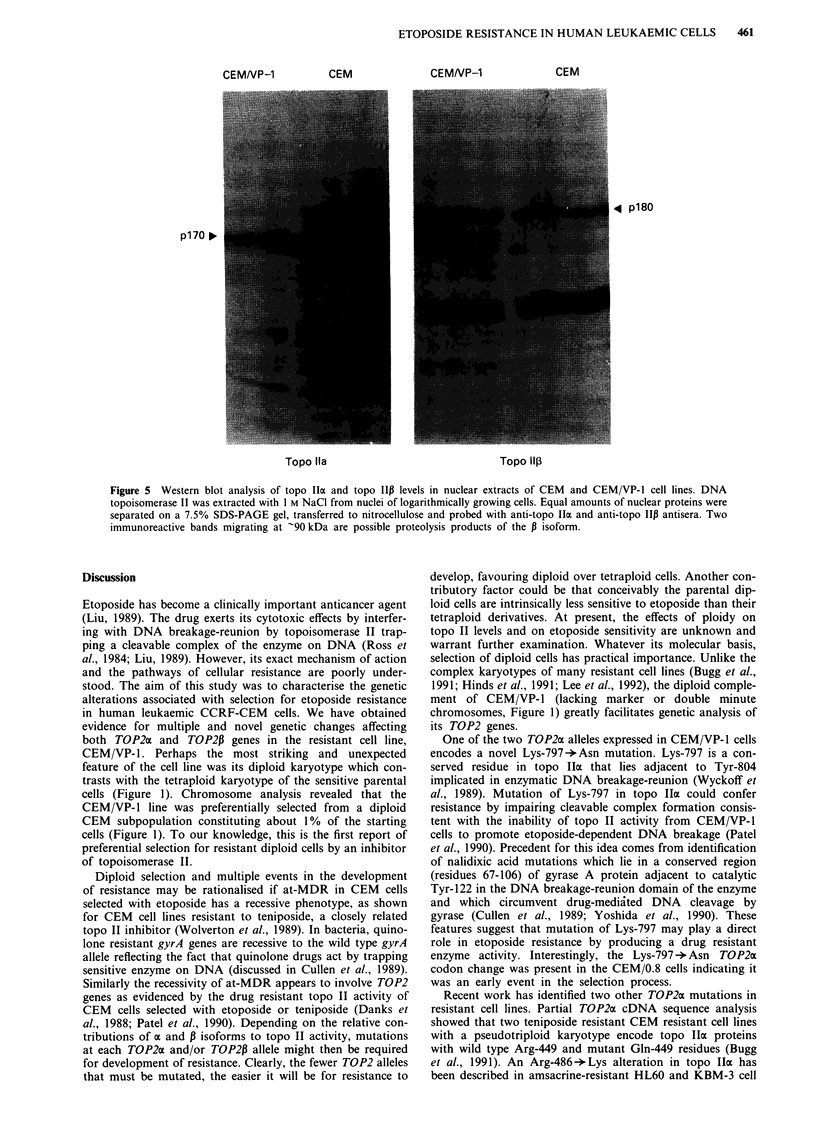
We have studied the genetic alterations acquired during selection of a cloned human leukaemic cell line (CEM/VP-1) that is 15-fold more resistant to the anticancer topoisomerase II-inhibitor etoposide than parental CCRF-CEM cells. CEM/VP-1 cells exhibit an 'atypical MDR' phenotype: cross resistance to other topo II inhibitors (but not Vinca alkaloids) and expression of a drug-resistant topo II activity. Cytogenetic and molecular studies revealed that the cell line carried multiple genetic changes affecting TOP2 genes encoding both topo II alpha and beta isoforms. CEM/VP-1 was diploid, 47,XX,+20, and appears to have been preferentially selected from a 1% diploid subpopulation present in the tetraploid parental cells. The same chromosomal abnormalities were present in resistant and sensitive cells except for an acquired 3p- change most likely deleting one TOP2 beta allele. PCR/DNA sequence analysis and allele-specific hybridisation showed that one of two TOP2 alpha alleles expressed in CEM/VP-1 cells had acquired a Lys-797-->Asn codon change. This mutation lies close to the catalytic Tyr-804 residue of the protein and may interfere with drug-induced trapping of the cleavable complex. Alternatively, it could exert a loss of function phenotype. CEM/VP-1 cells did not exhibit codon 449 or 486 TOP2 alpha mutations in the ATP binding domain reported in two other resistant cell lines. Diploid selection and multiple changes observed in CEM/VP-1 cells appear to be consequences of the recessive phenotype of at-MDR. These results may be useful in approaching the mechanisms of clinical resistance.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin C. A., Fisher L. M. Isolation and characterization of a human cDNA clone encoding a novel DNA topoisomerase II homologue from HeLa cells. FEBS Lett. 1990 Jun 18;266(1-2):115–117. doi: 10.1016/0014-5793(90)81520-x. [DOI] [PubMed] [Google Scholar]
- Beck W. T., Cirtain M. C., Danks M. K., Felsted R. L., Safa A. R., Wolverton J. S., Suttle D. P., Trent J. M. Pharmacological, molecular, and cytogenetic analysis of "atypical" multidrug-resistant human leukemic cells. Cancer Res. 1987 Oct 15;47(20):5455–5460. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bugg B. Y., Danks M. K., Beck W. T., Suttle D. P. Expression of a mutant DNA topoisomerase II in CCRF-CEM human leukemic cells selected for resistance to teniposide. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7654–7658. doi: 10.1073/pnas.88.17.7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charcosset J. Y., Saucier J. M., Jacquemin-Sablon A. Reduced DNA topoisomerase II activity and drug-stimulated DNA cleavage in 9-hydroxyellipticine resistant cells. Biochem Pharmacol. 1988 Jun 1;37(11):2145–2149. doi: 10.1016/0006-2952(88)90573-4. [DOI] [PubMed] [Google Scholar]
- Chen G. L., Yang L., Rowe T. C., Halligan B. D., Tewey K. M., Liu L. F. Nonintercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Nov 10;259(21):13560–13566. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chung T. D., Drake F. H., Tan K. B., Per S. R., Crooke S. T., Mirabelli C. K. Characterization and immunological identification of cDNA clones encoding two human DNA topoisomerase II isozymes. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9431–9435. doi: 10.1073/pnas.86.23.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen M. E., Wyke A. W., Kuroda R., Fisher L. M. Cloning and characterization of a DNA gyrase A gene from Escherichia coli that confers clinical resistance to 4-quinolones. Antimicrob Agents Chemother. 1989 Jun;33(6):886–894. doi: 10.1128/aac.33.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danks M. K., Schmidt C. A., Cirtain M. C., Suttle D. P., Beck W. T. Altered catalytic activity of and DNA cleavage by DNA topoisomerase II from human leukemic cells selected for resistance to VM-26. Biochemistry. 1988 Nov 29;27(24):8861–8869. doi: 10.1021/bi00424a026. [DOI] [PubMed] [Google Scholar]
- Danks M. K., Yalowich J. C., Beck W. T. Atypical multiple drug resistance in a human leukemic cell line selected for resistance to teniposide (VM-26). Cancer Res. 1987 Mar 1;47(5):1297–1301. [PubMed] [Google Scholar]
- Drake F. H., Hofmann G. A., Bartus H. F., Mattern M. R., Crooke S. T., Mirabelli C. K. Biochemical and pharmacological properties of p170 and p180 forms of topoisomerase II. Biochemistry. 1989 Oct 3;28(20):8154–8160. doi: 10.1021/bi00446a029. [DOI] [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Estey E. H., Silberman L., Beran M., Andersson B. S., Zwelling L. A. The interaction between nuclear topoisomerase II activity from human leukemia cells, exogenous DNA, and 4'-(9-acridinylamino)methanesulfon-m-anisidide (m-AMSA) or 4-(4,6-O-ethylidene-beta-D-glucopyranoside) (VP-16) indicates the sensitivity of the cells to the drugs. Biochem Biophys Res Commun. 1987 Apr 29;144(2):787–793. doi: 10.1016/s0006-291x(87)80033-5. [DOI] [PubMed] [Google Scholar]
- Glisson B., Gupta R., Smallwood-Kentro S., Ross W. Characterization of acquired epipodophyllotoxin resistance in a Chinese hamster ovary cell line: loss of drug-stimulated DNA cleavage activity. Cancer Res. 1986 Apr;46(4 Pt 2):1934–1938. [PubMed] [Google Scholar]
- Hinds M., Deisseroth K., Mayes J., Altschuler E., Jansen R., Ledley F. D., Zwelling L. A. Identification of a point mutation in the topoisomerase II gene from a human leukemia cell line containing an amsacrine-resistant form of topoisomerase II. Cancer Res. 1991 Sep 1;51(17):4729–4731. [PubMed] [Google Scholar]
- Huff A. C., Ward R. E., 4th, Kreuzer K. N. Mutational alteration of the breakage/resealing subunit of bacteriophage T4 DNA topoisomerase confers resistance to antitumor agent m-AMSA. Mol Gen Genet. 1990 Mar;221(1):27–32. doi: 10.1007/BF00280363. [DOI] [PubMed] [Google Scholar]
- Kasahara K., Fujiwara Y., Sugimoto Y., Nishio K., Tamura T., Matsuda T., Saijo N. Determinants of response to the DNA topoisomerase II inhibitors doxorubicin and etoposide in human lung cancer cell lines. J Natl Cancer Inst. 1992 Jan 15;84(2):113–118. doi: 10.1093/jnci/84.2.113. [DOI] [PubMed] [Google Scholar]
- Lee M. S., Wang J. C., Beran M. Two independent amsacrine-resistant human myeloid leukemia cell lines share an identical point mutation in the 170 kDa form of human topoisomerase II. J Mol Biol. 1992 Feb 20;223(4):837–843. doi: 10.1016/0022-2836(92)90245-f. [DOI] [PubMed] [Google Scholar]
- Liu L. F. DNA topoisomerase poisons as antitumor drugs. Annu Rev Biochem. 1989;58:351–375. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- Moscow J. A., Cowan K. H. Multidrug resistance. J Natl Cancer Inst. 1988 Mar 2;80(1):14–20. doi: 10.1093/jnci/80.1.14. [DOI] [PubMed] [Google Scholar]
- Nelson E. M., Tewey K. M., Liu L. F. Mechanism of antitumor drug action: poisoning of mammalian DNA topoisomerase II on DNA by 4'-(9-acridinylamino)-methanesulfon-m-anisidide. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1361–1365. doi: 10.1073/pnas.81.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S., Austin C. A., Fisher L. M. Development and properties of an etoposide-resistant human leukaemic CCRF-CEM cell line. Anticancer Drug Des. 1990 Feb;5(1):149–157. [PubMed] [Google Scholar]
- Pommier Y., Schwartz R. E., Zwelling L. A., Kerrigan D., Mattern M. R., Charcosset J. Y., Jacquemin-Sablon A., Kohn K. W. Reduced formation of protein-associated DNA strand breaks in Chinese hamster cells resistant to topoisomerase II inhibitors. Cancer Res. 1986 Feb;46(2):611–616. [PubMed] [Google Scholar]
- Ross W. E., Glaubiger D. L., Kohn K. W. Protein-associated DNA breaks in cells treated with adriamycin or ellipticine. Biochim Biophys Acta. 1978 Jun 22;519(1):23–30. doi: 10.1016/0005-2787(78)90059-x. [DOI] [PubMed] [Google Scholar]
- Ross W., Rowe T., Glisson B., Yalowich J., Liu L. Role of topoisomerase II in mediating epipodophyllotoxin-induced DNA cleavage. Cancer Res. 1984 Dec;44(12 Pt 1):5857–5860. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. B., Dorman T. E., Falls K. M., Chung T. D., Mirabelli C. K., Crooke S. T., Mao J. Topoisomerase II alpha and topoisomerase II beta genes: characterization and mapping to human chromosomes 17 and 3, respectively. Cancer Res. 1992 Jan 1;52(1):231–234. [PubMed] [Google Scholar]
- Tewey K. M., Chen G. L., Nelson E. M., Liu L. F. Intercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Jul 25;259(14):9182–9187. [PubMed] [Google Scholar]
- Trent J. M., Thompson F. H. Methods for chromosome banding of human and experimental tumors in vitro. Methods Enzymol. 1987;151:267–279. doi: 10.1016/s0076-6879(87)51023-0. [DOI] [PubMed] [Google Scholar]
- Tsai-Pflugfelder M., Liu L. F., Liu A. A., Tewey K. M., Whang-Peng J., Knutsen T., Huebner K., Croce C. M., Wang J. C. Cloning and sequencing of cDNA encoding human DNA topoisomerase II and localization of the gene to chromosome region 17q21-22. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7177–7181. doi: 10.1073/pnas.85.19.7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A. Tumor suppressor genes. Science. 1991 Nov 22;254(5035):1138–1146. doi: 10.1126/science.1659741. [DOI] [PubMed] [Google Scholar]
- Wolverton J. S., Danks M. K., Schmidt C. A., Beck W. T. Genetic characterization of the multidrug-resistant phenotype of VM-26-resistant human leukemic cells. Cancer Res. 1989 May 1;49(9):2422–2426. [PubMed] [Google Scholar]
- Wyckoff E., Natalie D., Nolan J. M., Lee M., Hsieh T. Structure of the Drosophila DNA topoisomerase II gene. Nucleotide sequence and homology among topoisomerases II. J Mol Biol. 1989 Jan 5;205(1):1–13. doi: 10.1016/0022-2836(89)90361-6. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Bogaki M., Nakamura M., Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob Agents Chemother. 1990 Jun;34(6):1271–1272. doi: 10.1128/aac.34.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Bogaki M., Nakamura M., Yamanaka L. M., Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrB gene of Escherichia coli. Antimicrob Agents Chemother. 1991 Aug;35(8):1647–1650. doi: 10.1128/aac.35.8.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwelling L. A., Michaels S., Erickson L. C., Ungerleider R. S., Nichols M., Kohn K. W. Protein-associated deoxyribonucleic acid strand breaks in L1210 cells treated with the deoxyribonucleic acid intercalating agents 4'-(9-acridinylamino) methanesulfon-m-anisidide and adriamycin. Biochemistry. 1981 Nov 10;20(23):6553–6563. doi: 10.1021/bi00526a006. [DOI] [PubMed] [Google Scholar]