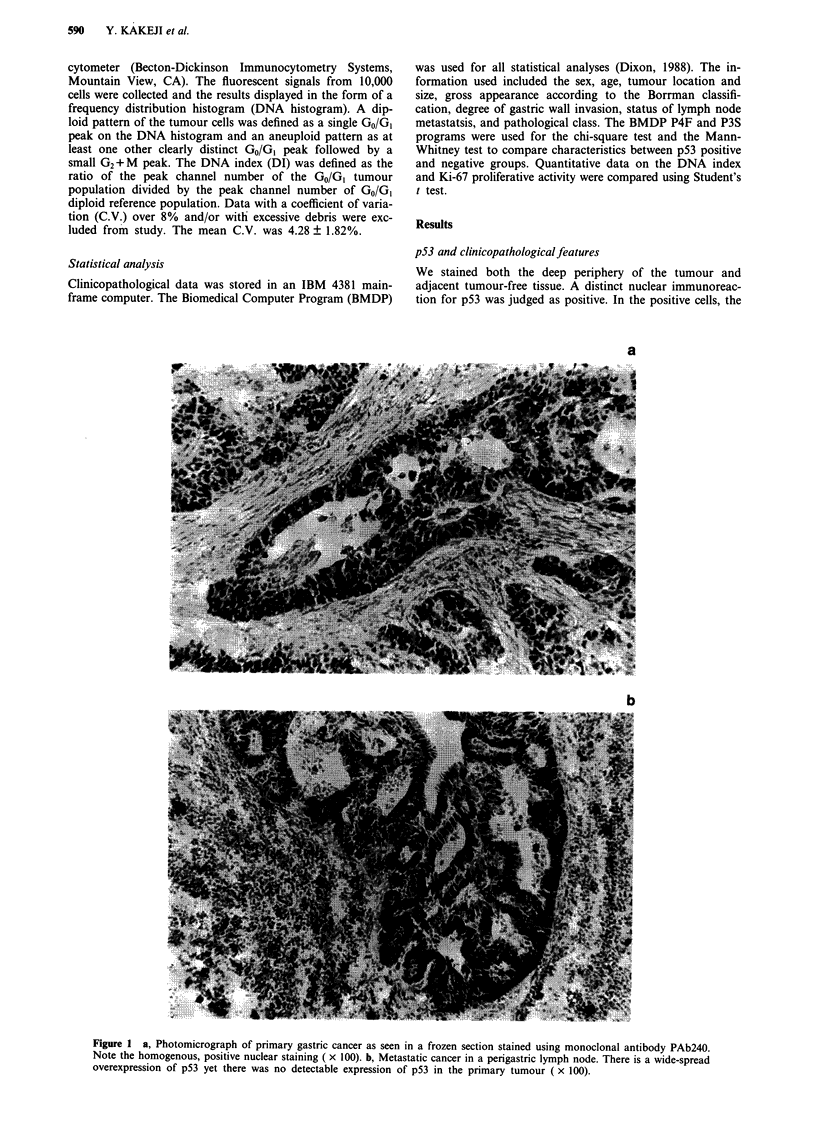
Abstract
Overexpression of the tumour suppressor gene p53 was investigated immunohistochemically in 96 primary gastric carcinomas and 26 corresponding metastatic perigastric lymph nodes. Abnormalities in p53 expression were found in 52 (54%) of the 96 primary carcinomas. Tumours stained positively for p53 frequently metastasised to lymph nodes (the metastatic rate: 85%) compared to findings in those with negative p53 staining (64%, P < 0.05). Ninety-two percent (24/26) of the malignant cells in the lymph nodes stained positively for p53. When the DNA ploidy pattern of the tumour was determined by flow cytometry, the aneuploid tumours in p53 positive and negative groups accounted for 69% and 45%, respectively (P < 0.05). Proliferative activity of the tumour, as measured by Ki-67 labelling, was significantly higher (30.6 +/- 12.0%) in the p53 positive group than that (25.1 +/- 10.7%) in the p53 negative group (P < 0.05). Thus, gastric cancer with a mutant p53 has high proliferative activity and metastasis to lymph nodes will probably occur.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartek J., Iggo R., Gannon J., Lane D. P. Genetic and immunochemical analysis of mutant p53 in human breast cancer cell lines. Oncogene. 1990 Jun;5(6):893–899. [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Cairns J. The origin of human cancers. Nature. 1981 Jan 29;289(5796):353–357. doi: 10.1038/289353a0. [DOI] [PubMed] [Google Scholar]
- Cattoretti G., Rilke F., Andreola S., D'Amato L., Delia D. P53 expression in breast cancer. Int J Cancer. 1988 Feb 15;41(2):178–183. doi: 10.1002/ijc.2910410204. [DOI] [PubMed] [Google Scholar]
- Davidoff A. M., Herndon J. E., 2nd, Glover N. S., Kerns B. J., Pence J. C., Iglehart J. D., Marks J. R. Relation between p53 overexpression and established prognostic factors in breast cancer. Surgery. 1991 Aug;110(2):259–264. [PubMed] [Google Scholar]
- Davidoff A. M., Humphrey P. A., Iglehart J. D., Marks J. R. Genetic basis for p53 overexpression in human breast cancer. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):5006–5010. doi: 10.1073/pnas.88.11.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Tan T. H., Eliyahu D., Oren M., Levine A. J. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol. 1988 Feb;8(2):531–539. doi: 10.1128/mcb.8.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon J. V., Greaves R., Iggo R., Lane D. P. Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J. 1990 May;9(5):1595–1602. doi: 10.1002/j.1460-2075.1990.tb08279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J., Schwab U., Lemke H., Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983 Jan 15;31(1):13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- Hiyoshi H., Matsuno Y., Kato H., Shimosato Y., Hirohashi S. Clinicopathological significance of nuclear accumulation of tumor suppressor gene p53 product in primary lung cancer. Jpn J Cancer Res. 1992 Jan;83(1):101–106. doi: 10.1111/j.1349-7006.1992.tb02358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth R. E., Lee W. H. Tumor suppressor genes: new prospects for cancer research. J Natl Cancer Inst. 1991 Jan 16;83(2):91–96. doi: 10.1093/jnci/83.2.91. [DOI] [PubMed] [Google Scholar]
- Iggo R., Gatter K., Bartek J., Lane D., Harris A. L. Increased expression of mutant forms of p53 oncogene in primary lung cancer. Lancet. 1990 Mar 24;335(8691):675–679. doi: 10.1016/0140-6736(90)90801-b. [DOI] [PubMed] [Google Scholar]
- Isola J., Visakorpi T., Holli K., Kallioniemi O. P. Association of overexpression of tumor suppressor protein p53 with rapid cell proliferation and poor prognosis in node-negative breast cancer patients. J Natl Cancer Inst. 1992 Jul 15;84(14):1109–1114. doi: 10.1093/jnci/84.14.1109. [DOI] [PubMed] [Google Scholar]
- Iwaya K., Tsuda H., Hiraide H., Tamaki K., Tamakuma S., Fukutomi T., Mukai K., Hirohashi S. Nuclear p53 immunoreaction associated with poor prognosis of breast cancer. Jpn J Cancer Res. 1991 Jul;82(7):835–840. doi: 10.1111/j.1349-7006.1991.tb02710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakeji Y., Korenaga D., Tsujitani S., Haraguchi M., Maehara Y., Sugimachi K. Predictive value of Ki-67 and argyrophilic nucleolar organizer region staining for lymph node metastasis in gastric cancer. Cancer Res. 1991 Jul 1;51(13):3503–3506. [PubMed] [Google Scholar]
- Kim J. H., Takahashi T., Chiba I., Park J. G., Birrer M. J., Roh J. K., De Lee H., Kim J. P., Minna J. D., Gazdar A. F. Occurrence of p53 gene abnormalities in gastric carcinoma tumors and cell lines. J Natl Cancer Inst. 1991 Jul 3;83(13):938–943. doi: 10.1093/jnci/83.13.938. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Mercer W. E., Avignolo C., Baserga R. Role of the p53 protein in cell proliferation as studied by microinjection of monoclonal antibodies. Mol Cell Biol. 1984 Feb;4(2):276–281. doi: 10.1128/mcb.4.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro J. M., Baker S. J., Preisinger A. C., Jessup J. M., Hostetter R., Cleary K., Bigner S. H., Davidson N., Baylin S., Devilee P. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Rodrigues N. R., Rowan A., Smith M. E., Kerr I. B., Bodmer W. F., Gannon J. V., Lane D. P. p53 mutations in colorectal cancer. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7555–7559. doi: 10.1073/pnas.87.19.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K., Murakami T., Murakami T., Nakamura M. Intratumoral heterogeneity in DNA ploidy of esophageal squamous cell carcinomas. Cancer. 1991 Dec 1;68(11):2403–2406. doi: 10.1002/1097-0142(19911201)68:11<2403::aid-cncr2820681112>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Scott N., Sagar P., Stewart J., Blair G. E., Dixon M. F., Quirke P. p53 in colorectal cancer: clinicopathological correlation and prognostic significance. Br J Cancer. 1991 Feb;63(2):317–319. doi: 10.1038/bjc.1991.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seruca R., David L., Holm R., Nesland J. M., Fangan B. M., Castedo S., Sobrinho-Simões M., Børresen A. L. P53 mutations in gastric carcinomas. Br J Cancer. 1992 May;65(5):708–710. doi: 10.1038/bjc.1992.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasawa S., Urabe K., Yanagawa Y., Toshitani K., Iwama T., Sasazuki T. p53 gene mutations in colorectal tumors from patients with familial polyposis coli. Cancer Res. 1991 Jun 1;51(11):2874–2878. [PubMed] [Google Scholar]
- Shohat O., Greenberg M., Reisman D., Oren M., Rotter V. Inhibition of cell growth mediated by plasmids encoding p53 anti-sense. Oncogene. 1987;1(3):277–283. [PubMed] [Google Scholar]
- Tamura G., Kihana T., Nomura K., Terada M., Sugimura T., Hirohashi S. Detection of frequent p53 gene mutations in primary gastric cancer by cell sorting and polymerase chain reaction single-strand conformation polymorphism analysis. Cancer Res. 1991 Jun 1;51(11):3056–3058. [PubMed] [Google Scholar]
- Yamada Y., Yoshida T., Hayashi K., Sekiya T., Yokota J., Hirohashi S., Nakatani K., Nakano H., Sugimura T., Terada M. p53 gene mutations in gastric cancer metastases and in gastric cancer cell lines derived from metastases. Cancer Res. 1991 Nov 1;51(21):5800–5805. [PubMed] [Google Scholar]
- Yasui W., Yoshida K., Ito H., Tahara E. [Molecular diagnosis of gastric cancer]. Gan To Kagaku Ryoho. 1991 Jan;18(1):7–13. [PubMed] [Google Scholar]