Abstract
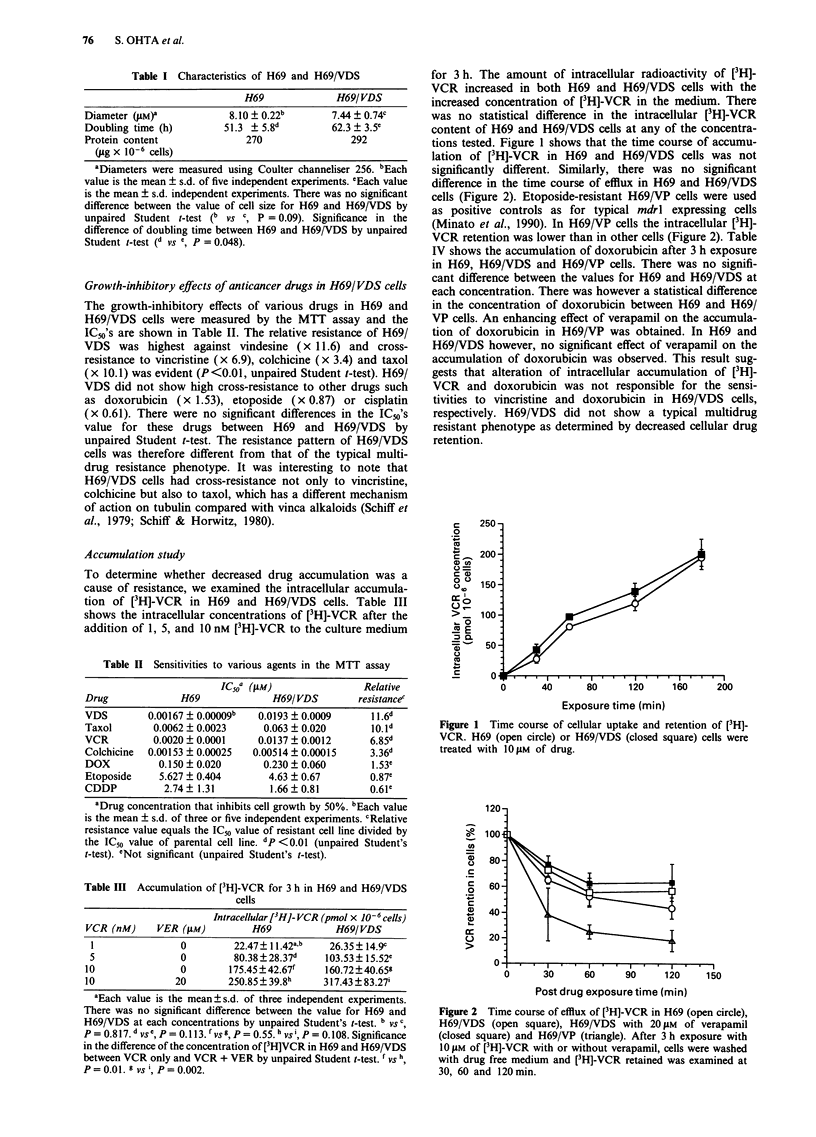
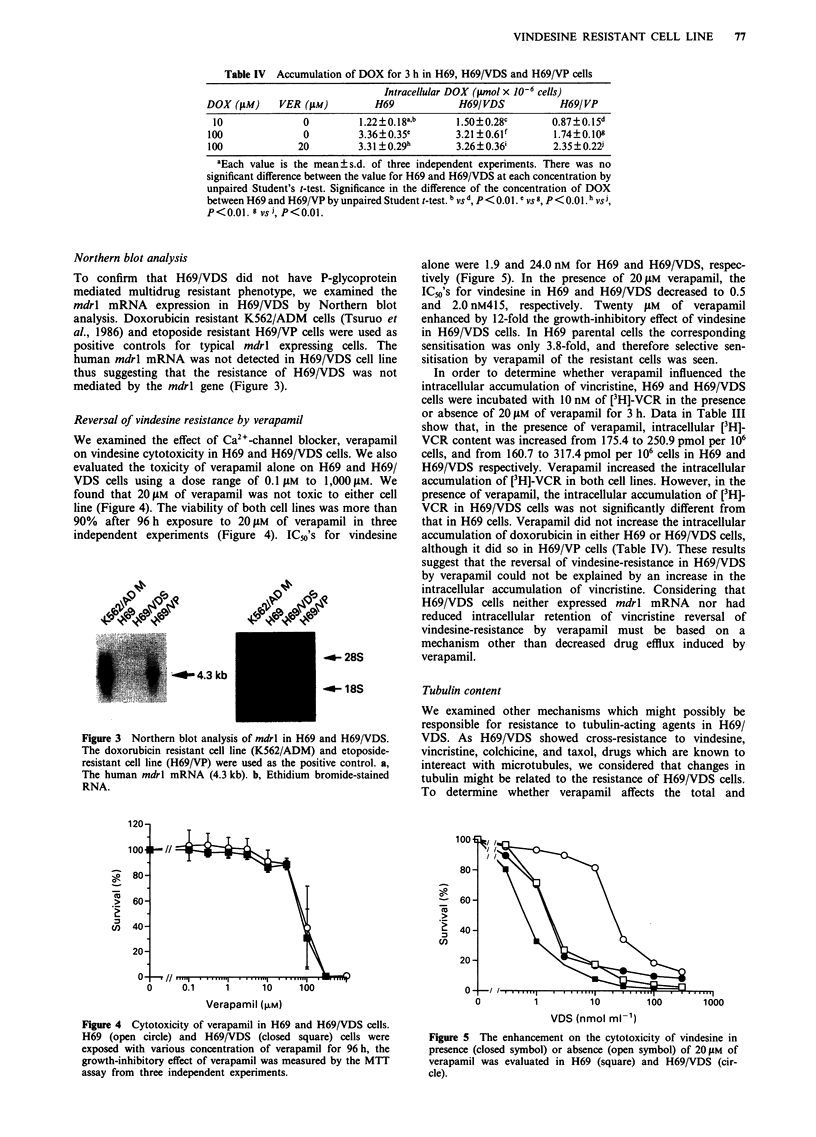
We established a vindesine-resistant (x 11.6) human small-cell lung cancer cell line (H69/VDS) by stepwise exposure of parent line H69 to vindesine. H69/VDS showed cross-resistance to taxol (x 10.1), vincristine (x 6.9) and colchicine (x 3.4) but not to doxorubicin, cisplatin or etoposide. There was no significant difference in intracellular [3H]-vincristine and doxorubicin accumulation between H69 and H69/VDS cells. The human mdr1 mRNA was not detected in either of the cell lines. These results indicated that H69/VDS did not express a typical multidrug resistant phenotype. Addition of 20 microM verapamil enhanced the growth inhibitory effect of vindesine on both H69/VDS (x 12.0) and H69 cells (x 3.8). The amount of total tubulin in H69/VDS cells was lower than that in the H69 parental cells. No significant increase was observed in the amount of total and polymerised tubulins of H69 cells. In H69/VDS cells, however, verapamil increased the amount of total tubulin to the level of parental cells, but decreased the amount of polymerised tubulin. Modulation of tubulin may play a role in the resistance to vindesine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck W. T. The cell biology of multiple drug resistance. Biochem Pharmacol. 1987 Sep 15;36(18):2879–2887. doi: 10.1016/0006-2952(87)90198-5. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Horichi N., Tapiero H., Sugimoto Y., Bungo M., Nishiyama M., Fourcade A., Lampidis T. J., Kasahara K., Sasaki Y., Takahashi T. 3'-Deamino-3'-morpholino-13-deoxo-10-hydroxycarminomycin conquers multidrug resistance by rapid influx following higher frequency of formation of DNA single- and double-strand breaks. Cancer Res. 1990 Aug 1;50(15):4698–4701. [PubMed] [Google Scholar]
- Jordan M. A., Thrower D., Wilson L. Mechanism of inhibition of cell proliferation by Vinca alkaloids. Cancer Res. 1991 Apr 15;51(8):2212–2222. [PubMed] [Google Scholar]
- Lai S. L., Goldstein L. J., Gottesman M. M., Pastan I., Tsai C. M., Johnson B. E., Mulshine J. L., Ihde D. C., Kayser K., Gazdar A. F. MDR1 gene expression in lung cancer. J Natl Cancer Inst. 1989 Aug 2;81(15):1144–1150. doi: 10.1093/jnci/81.15.1144. [DOI] [PubMed] [Google Scholar]
- Lee W. C., Lin K. Y., Chen K. D., Lai Y. K. Induction of HSP70 is associated with vincristine resistance in heat-shocked 9L rat brain tumour cells. Br J Cancer. 1992 Oct;66(4):653–659. doi: 10.1038/bjc.1992.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L., Hall C., Leung T., Whatley S. The relationship of the rat brain 68 kDa microtubule-associated protein with synaptosomal plasma membranes and with the Drosophila 70 kDa heat-shock protein. Biochem J. 1984 Dec 1;224(2):677–680. doi: 10.1042/bj2240677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minato K., Kanzawa F., Nishio K., Nakagawa K., Fujiwara Y., Saijo N. Characterization of an etoposide-resistant human small-cell lung cancer cell line. Cancer Chemother Pharmacol. 1990;26(5):313–317. doi: 10.1007/BF02897284. [DOI] [PubMed] [Google Scholar]
- Minotti A. M., Barlow S. B., Cabral F. Resistance to antimitotic drugs in Chinese hamster ovary cells correlates with changes in the level of polymerized tubulin. J Biol Chem. 1991 Feb 25;266(6):3987–3994. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nygren P., Larsson R. Verapamil and cyclosporin A sensitize human kidney tumor cells to vincristine in absence of membrane P-glycoprotein and without apparent changes in the cytoplasmic free Ca2+ concentration. Biosci Rep. 1990 Apr;10(2):231–237. doi: 10.1007/BF01116583. [DOI] [PubMed] [Google Scholar]
- Roninson I. B., Chin J. E., Choi K. G., Gros P., Housman D. E., Fojo A., Shen D. W., Gottesman M. M., Pastan I. Isolation of human mdr DNA sequences amplified in multidrug-resistant KB carcinoma cells. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4538–4542. doi: 10.1073/pnas.83.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S. N., Horwitz S. B. A phosphoglycoprotein associated with taxol resistance in J774.2 cells. Cancer Res. 1985 Aug;45(8):3856–3863. [PubMed] [Google Scholar]
- Schiff P. B., Fant J., Horwitz S. B. Promotion of microtubule assembly in vitro by taxol. Nature. 1979 Feb 22;277(5698):665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- Schiff P. B., Horwitz S. B. Taxol assembles tubulin in the absence of exogenous guanosine 5'-triphosphate or microtubule-associated proteins. Biochemistry. 1981 May 26;20(11):3247–3252. doi: 10.1021/bi00514a041. [DOI] [PubMed] [Google Scholar]
- Schiff P. B., Horwitz S. B. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrower D., Jordan M. A., Wilson L. Quantitation of cellular tubulin in microtubules and tubulin pools by a competitive ELISA. J Immunol Methods. 1991 Jan 24;136(1):45–51. doi: 10.1016/0022-1759(91)90248-e. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruo T., Iida-Saito H., Kawabata H., Oh-hara T., Hamada H., Utakoji T. Characteristics of resistance to adriamycin in human myelogenous leukemia K562 resistant to adriamycin and in isolated clones. Jpn J Cancer Res. 1986 Jul;77(7):682–692. [PubMed] [Google Scholar]
- Versantvoort C. H., Broxterman H. J., Pinedo H. M., de Vries E. G., Feller N., Kuiper C. M., Lankelma J. Energy-dependent processes involved in reduced drug accumulation in multidrug-resistant human lung cancer cell lines without P-glycoprotein expression. Cancer Res. 1992 Jan 1;52(1):17–23. [PubMed] [Google Scholar]
- Yusa K., Tsuruo T. Reversal mechanism of multidrug resistance by verapamil: direct binding of verapamil to P-glycoprotein on specific sites and transport of verapamil outward across the plasma membrane of K562/ADM cells. Cancer Res. 1989 Sep 15;49(18):5002–5006. [PubMed] [Google Scholar]