Abstract
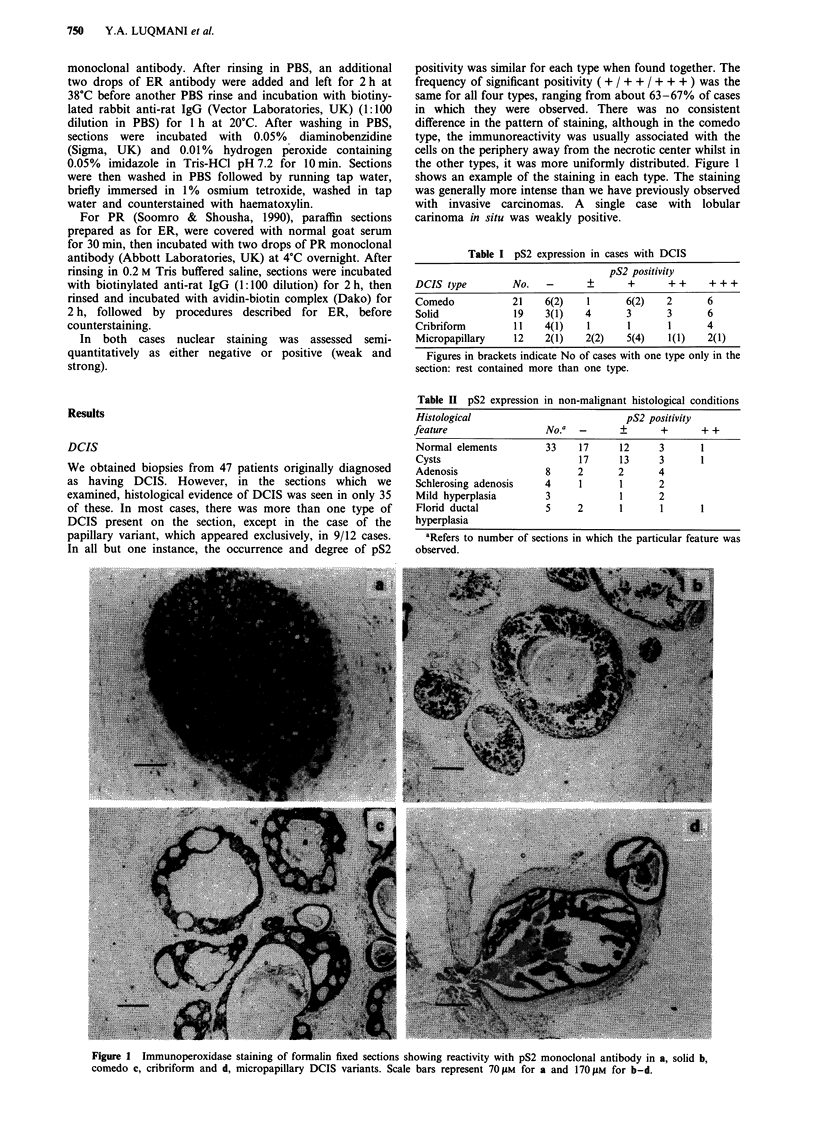
The expression of pS2 was examined histochemically in paraffin sections taken from biopsy material from patients diagnosed with ductal carcinoma in situ (DCIS). Often intense immunoreactivity, to an anti-pS2 monoclonal antibody, was observed in comedo, solid, cribriform and micropapillary types of DCIS, with significant positivity found in 63-67% of cases. In 15 samples analysed, we found a good correlation between pS2 expression and presence of progesterone receptor positive cells, but not with estrogen receptor. There was only a limited degree of correspondence between the cells staining with these anti-sera. Some pS2 positive cells were also seen in normal acini in areas adjacent to cancer but much less frequently in sections of normal breast from reduction mammoplasty. Most normal areas were negative, as were cysts. In benign proliferative conditions (seen in sections with and without DCIS) such as adenosis, sclerosing adenosis, mild and florid ductal epithelial hyperplasia, significant pS2 positivity was seen in about 50% of cases. These results suggest that there is a progressive increase in pS2 from normal to benign to cancer cells and that this gene is expressed in both the invasive and pre-invasive forms of breast cancer.
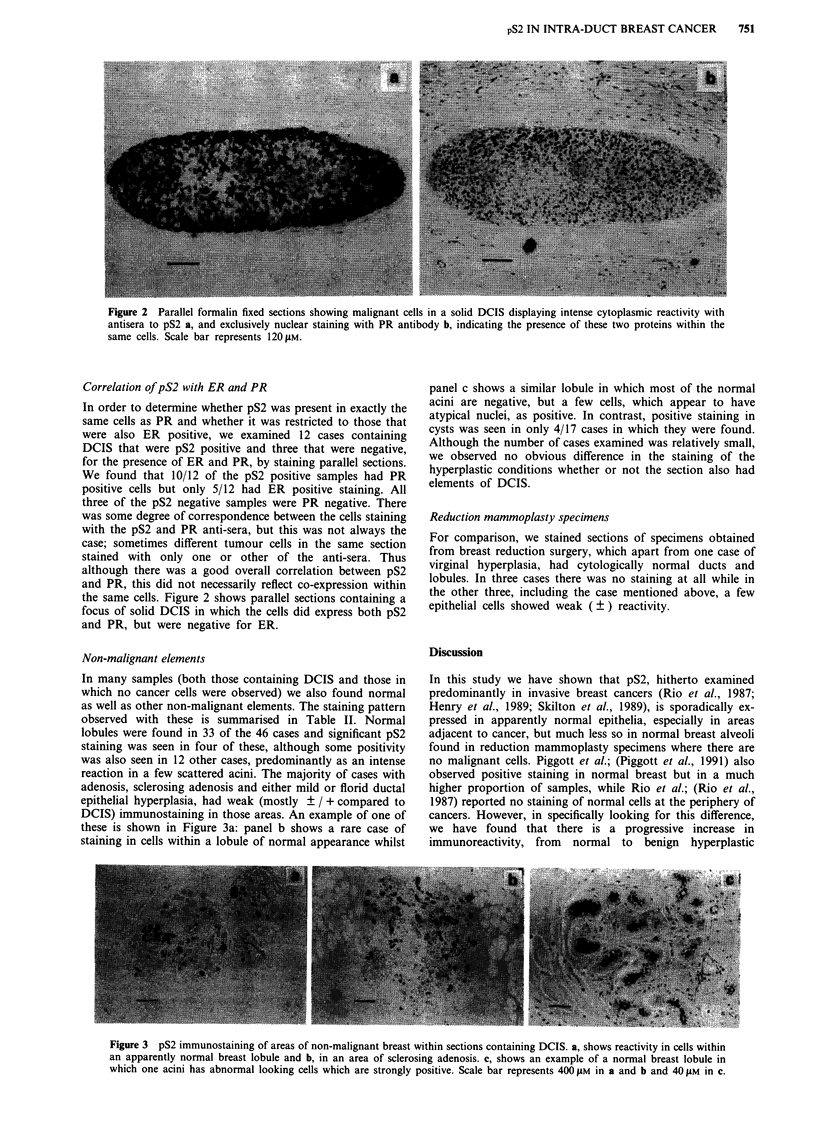
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartkova J., Barnes D. M., Millis R. R., Gullick W. J. Immunohistochemical demonstration of c-erbB-2 protein in mammary ductal carcinoma in situ. Hum Pathol. 1990 Nov;21(11):1164–1167. doi: 10.1016/0046-8177(90)90154-w. [DOI] [PubMed] [Google Scholar]
- Campbell T., Skilton R. A., Coombes R. C., Shousha S., Graham M. D., Luqmani Y. A. Isolation of a lactoferrin cDNA clone and its expression in human breast cancer. Br J Cancer. 1992 Jan;65(1):19–26. doi: 10.1038/bjc.1992.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusebi V., Collina G., Bussolati G. Carcinoma in situ in sclerosing adenosis of the breast: an immunocytochemical study. Semin Diagn Pathol. 1989 May;6(2):146–152. [PubMed] [Google Scholar]
- Fentiman I. S. Treatment of screen detected ductal carcinoma in situ: a silver lining within a grey cloud? Br J Cancer. 1990 Jun;61(6):795–796. doi: 10.1038/bjc.1990.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuqua S. A., Fitzgerald S. D., Chamness G. C., Tandon A. K., McDonnell D. P., Nawaz Z., O'Malley B. W., McGuire W. L. Variant human breast tumor estrogen receptor with constitutive transcriptional activity. Cancer Res. 1991 Jan 1;51(1):105–109. [PubMed] [Google Scholar]
- Giri D. D., Dundas S. A., Nottingham J. F., Underwood J. C. Oestrogen receptors in benign epithelial lesions and intraduct carcinomas of the breast: an immunohistological study. Histopathology. 1989 Dec;15(6):575–584. doi: 10.1111/j.1365-2559.1989.tb01623.x. [DOI] [PubMed] [Google Scholar]
- Henry J. A., Bennett M. K., Piggott N. H., Levett D. L., May F. E., Westley B. R. Expression of the pNR-2/pS2 protein in diverse human epithelial tumours. Br J Cancer. 1991 Oct;64(4):677–682. doi: 10.1038/bjc.1991.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J. A., Nicholson S., Hennessy C., Lennard T. W., May F. E., Westley B. R. Expression of the oestrogen regulated pNR-2 mRNA in human breast cancer: relation to oestrogen receptor mRNA levels and response to tamoxifen therapy. Br J Cancer. 1990 Jan;61(1):32–38. doi: 10.1038/bjc.1990.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A., Page D. L., Dupont W. D., Rogers L. W. Invasive breast cancer risk in women with sclerosing adenosis. Cancer. 1989 Nov 15;64(10):1977–1983. doi: 10.1002/1097-0142(19891115)64:10<1977::aid-cncr2820641002>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Luqmani Y. A., Ryall G., Shousha S., Coombes R. C. An immunohistochemical survey of pS2 expression in human epithelial cancers. Int J Cancer. 1992 Jan 21;50(2):302–304. doi: 10.1002/ijc.2910500222. [DOI] [PubMed] [Google Scholar]
- Luqmani Y., Bennett C., Paterson I., Corbishley C. M., Rio M. C., Chambon P., Ryall G. Expression of the pS2 gene in normal, benign and neoplastic human stomach. Int J Cancer. 1989 Nov 15;44(5):806–812. doi: 10.1002/ijc.2910440510. [DOI] [PubMed] [Google Scholar]
- Masiakowski P., Breathnach R., Bloch J., Gannon F., Krust A., Chambon P. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res. 1982 Dec 20;10(24):7895–7903. doi: 10.1093/nar/10.24.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. B., Bulbrook R. D. The epidemiology and etiology of breast cancer. N Engl J Med. 1980 Nov 20;303(21):1246–1248. doi: 10.1056/NEJM198011203032130. [DOI] [PubMed] [Google Scholar]
- Piggott N. H., Henry J. A., May F. E., Westley B. R. Antipeptide antibodies against the pNR-2 oestrogen-regulated protein of human breast cancer cells and detection of pNR-2 expression in normal tissues by immunohistochemistry. J Pathol. 1991 Feb;163(2):95–104. doi: 10.1002/path.1711630204. [DOI] [PubMed] [Google Scholar]
- Rio M. C., Bellocq J. P., Daniel J. Y., Tomasetto C., Lathe R., Chenard M. P., Batzenschlager A., Chambon P. Breast cancer-associated pS2 protein: synthesis and secretion by normal stomach mucosa. Science. 1988 Aug 5;241(4866):705–708. doi: 10.1126/science.3041593. [DOI] [PubMed] [Google Scholar]
- Rio M. C., Bellocq J. P., Gairard B., Rasmussen U. B., Krust A., Koehl C., Calderoli H., Schiff V., Renaud R., Chambon P. Specific expression of the pS2 gene in subclasses of breast cancers in comparison with expression of the estrogen and progesterone receptors and the oncogene ERBB2. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9243–9247. doi: 10.1073/pnas.84.24.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio M. C., Chenard M. P., Wolf C., Marcellin L., Tomasetto C., Lathe R., Bellocq J. P., Chambon P. Induction of pS2 and hSP genes as markers of mucosal ulceration of the digestive tract. Gastroenterology. 1991 Feb;100(2):375–379. doi: 10.1016/0016-5085(91)90205-y. [DOI] [PubMed] [Google Scholar]
- Rubin A. L. Suppression of transformation by and growth adaptation to low concentrations of glutamine in NIH-3T3 cells. Cancer Res. 1990 May 1;50(9):2832–2839. [PubMed] [Google Scholar]
- Schwartz L. H., Koerner F. C., Edgerton S. M., Sawicka J. M., Rio M. C., Bellocq J. P., Chambon P., Thor A. D. pS2 expression and response to hormonal therapy in patients with advanced breast cancer. Cancer Res. 1991 Jan 15;51(2):624–628. [PubMed] [Google Scholar]
- Seitz G., Thelsinger B., Tomasetto G., Rio M. C., Chambon P., Blin N., Welter G. Breast cancer-associated protein pS2 expression in tumors of the biliary tract. Am J Gastroenterol. 1991 Oct;86(10):1491–1494. [PubMed] [Google Scholar]
- Skilton R. A., Luqmani Y. A., McClelland R. A., Coombes R. C. Characterisation of a messenger RNA selectively expressed in human breast cancer. Br J Cancer. 1989 Aug;60(2):168–175. doi: 10.1038/bjc.1989.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soomro S., Shousha S. Demonstration of progesterone receptors in paraffin wax sections of breast carcinoma. J Clin Pathol. 1990 Aug;43(8):671–674. doi: 10.1136/jcp.43.8.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisinger B., Welter C., Seitz G., Rio M. C., Lathe R., Chambon P., Blin N. Expression of the breast cancer associated gene pS2 and the pancreatic spasmolytic polypeptide gene (hSP) in diffuse type of stomach carcinoma. Eur J Cancer. 1991;27(6):770–773. doi: 10.1016/0277-5379(91)90186-h. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Poulsom R., Stamp G. W., Hall P. A., Jeffery R. E., Longcroft J. M., Rio M. C., Tomasetto C., Chambon P. Epidermal growth factor (EGF/URO) induces expression of regulatory peptides in damaged human gastrointestinal tissues. J Pathol. 1990 Dec;162(4):279–284. doi: 10.1002/path.1711620402. [DOI] [PubMed] [Google Scholar]
- Wysocki S. J., Hahnel E., Masters A., Smith V., McCartney A. J., Hahnel R. Detection of pS2 messenger RNA in gynecological cancers. Cancer Res. 1990 Mar 15;50(6):1800–1802. [PubMed] [Google Scholar]