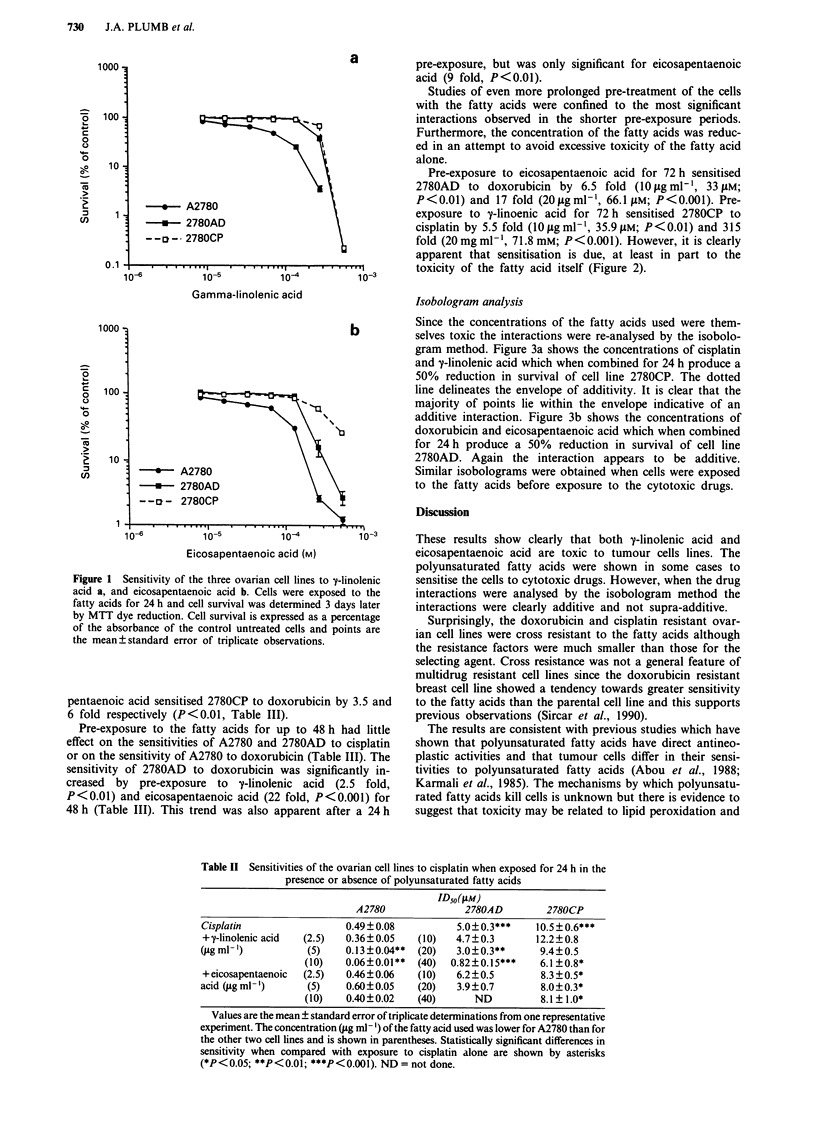
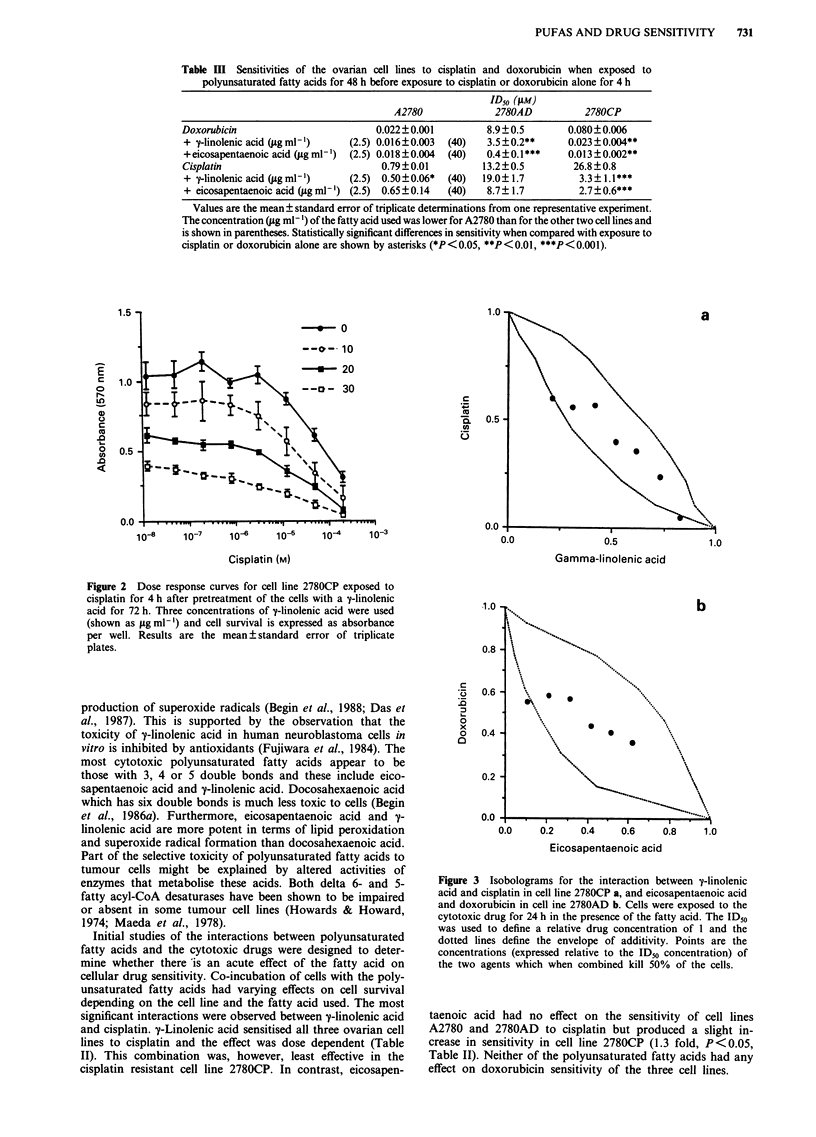
Abstract
Growth of cells in vitro in the presence of fatty acids can alter the membrane composition and hence fluidity and permeability. Exposure of both doxorubicin (2780AD) and cisplatin (2780CP) resistant human ovarian cell lines to non-toxic concentrations of polyunsaturated fatty acids (gamma-linolenic acid and eicosapentaenoic acid) either before or during exposure to the cytotoxic drug did not modulate drug sensitivity. However, the fatty acids were toxic in their own right. Whilst the ovarian cell lines 2780AD and 2780CP showed a small degree of cross resistance to both fatty acids the doxorubicin resistant breast cell line MCF7/Adr was slightly more sensitive than MCF7. When the interactions between the polyunsaturated fatty acids and cytotoxic drugs were analysed by the isobologram method the toxicities were shown to be additive. The combination of polyunsaturated fatty acids and cytotoxic drugs may have clinical potential provided that the normal tissue toxicities of the two treatments are not additive.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-el-Ela S. H., Prasse K. W., Carroll R., Wade A. E., Dharwadkar S., Bunce O. R. Eicosanoid synthesis in 7,12-dimethylbenz(a)anthracene-induced mammary carcinomas in Sprague-Dawley rats fed primrose oil, menhaden oil or corn oil diet. Lipids. 1988 Oct;23(10):948–954. doi: 10.1007/BF02536342. [DOI] [PubMed] [Google Scholar]
- Andrews P. A., Howell S. B. Cellular pharmacology of cisplatin: perspectives on mechanisms of acquired resistance. Cancer Cells. 1990 Feb;2(2):35–43. [PubMed] [Google Scholar]
- Basu A., Kozikowski A. P., Sato K., Lazo J. S. Cellular sensitization to cis-diamminedichloroplatinum(II) by novel analogues of the protein kinase C activator lyngbyatoxin A. Cancer Res. 1991 May 15;51(10):2511–2514. [PubMed] [Google Scholar]
- Beck S. A., Smith K. L., Tisdale M. J. Anticachectic and antitumor effect of eicosapentaenoic acid and its effect on protein turnover. Cancer Res. 1991 Nov 15;51(22):6089–6093. [PubMed] [Google Scholar]
- Booyens J., Engelbrecht P., le Roux S., Louwrens C. C., Van der Merwe C. F., Katzeff I. E. Some effects of the essential fatty acids linoleic acid and alpha-linolenic acid and of their metabolites gamma-linolenic acid, arachidonic acid, eicosapentaenoic acid, docosahexaenoic acid, and of prostaglandins A1 and E1 on the proliferation of human osteogenic sarcoma cells in culture. Prostaglandins Leukot Med. 1984 Jul;15(1):15–33. doi: 10.1016/0262-1746(84)90053-2. [DOI] [PubMed] [Google Scholar]
- Bégin M. E., Ells G., Das U. N., Horrobin D. F. Differential killing of human carcinoma cells supplemented with n-3 and n-6 polyunsaturated fatty acids. J Natl Cancer Inst. 1986 Nov;77(5):1053–1062. [PubMed] [Google Scholar]
- Bégin M. E., Ells G., Horrobin D. F. Polyunsaturated fatty acid-induced cytotoxicity against tumor cells and its relationship to lipid peroxidation. J Natl Cancer Inst. 1988 Apr 6;80(3):188–194. doi: 10.1093/jnci/80.3.188. [DOI] [PubMed] [Google Scholar]
- Carter W. H., Jr, Wampler G. L. Review of the application of response surface methodology in the combination therapy of cancer. Cancer Treat Rep. 1986 Jan;70(1):133–140. [PubMed] [Google Scholar]
- Church M. W., Dintcheff B. A., Gessner P. K. The interactive effects of alcohol and cocaine on maternal and fetal toxicity in the Long-Evans rat. Neurotoxicol Teratol. 1988 Jul-Aug;10(4):355–361. doi: 10.1016/0892-0362(88)90039-6. [DOI] [PubMed] [Google Scholar]
- Das U. N., Huang Y. S., Begin M. E., Ells G., Horrobin D. F. Uptake and distribution of cis-unsaturated fatty acids and their effect on free radical generation in normal and tumor cells in vitro. Free Radic Biol Med. 1987;3(1):9–14. doi: 10.1016/0891-5849(87)90033-5. [DOI] [PubMed] [Google Scholar]
- Eskimo diets and diseases. Lancet. 1983 May 21;1(8334):1139–1141. [PubMed] [Google Scholar]
- Fine R. L., Patel J., Chabner B. A. Phorbol esters induce multidrug resistance in human breast cancer cells. Proc Natl Acad Sci U S A. 1988 Jan;85(2):582–586. doi: 10.1073/pnas.85.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann J., Doppler W., Jakob A., Maly K., Posch L., Uberall F., Grunicke H. H. Enhancement of the antiproliferative effect of cis-diamminedichloroplatinum(II) and nitrogen mustard by inhibitors of protein kinase C. Int J Cancer. 1988 Sep 15;42(3):382–388. doi: 10.1002/ijc.2910420313. [DOI] [PubMed] [Google Scholar]
- Howard B. V., Howard W. J. Lipid metabolism in cultured cells. Adv Lipid Res. 1974;12(0):51–96. [PubMed] [Google Scholar]
- Isonishi S., Andrews P. A., Howell S. B. Increased sensitivity to cis-diamminedichloroplatinum(II) in human ovarian carcinoma cells in response to treatment with 12-O-tetradecanoylphorbol 13-acetate. J Biol Chem. 1990 Mar 5;265(7):3623–3627. [PubMed] [Google Scholar]
- Maeda M., Doi O., Akamatsu Y. Metabolic conversion of polyunsaturated fatty acids in mammalian cultured cells. Biochim Biophys Acta. 1978 Aug 25;530(2):153–164. [PubMed] [Google Scholar]
- Mann S. C., Andrews P. A., Howell S. B. Comparison of lipid content, surface membrane fluidity, and temperature dependence of cis-diamminedichloroplatinum(II) accumulation in sensitive and resistant human ovarian carcinoma cells. Anticancer Res. 1988 Nov-Dec;8(6):1211–1215. [PubMed] [Google Scholar]
- Peterson R. H., Meyers M. B., Spengler B. A., Biedler J. L. Alteration of plasma membrane glycopeptides and gangliosides of Chinese hamster cells accompanying development of resistance to daunorubicin and vincristine. Cancer Res. 1983 Jan;43(1):222–228. [PubMed] [Google Scholar]
- Plumb J. A., Milroy R., Kaye S. B. Effects of the pH dependence of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide-formazan absorption on chemosensitivity determined by a novel tetrazolium-based assay. Cancer Res. 1989 Aug 15;49(16):4435–4440. [PubMed] [Google Scholar]
- Ramu A., Glaubiger D., Magrath I. T., Joshi A. Plasma membrane lipid structural order in doxorubicin-sensitive and -resistant P388 cells. Cancer Res. 1983 Nov;43(11):5533–5537. [PubMed] [Google Scholar]
- Rintoul D. A., Center M. S. Involvement of plasma membrane lipid structural order in adriamycin resistance in Chinese hamster lung cells. Cancer Res. 1984 Nov;44(11):4978–4980. [PubMed] [Google Scholar]
- Siegfried J. A., Kennedy K. A., Sartorelli A. C., Tritton T. R. The role of membranes in the mechanism of action of the antineoplastic agent adriamycin. Spin-labeling studies with chronically hypoxic and drug-resistant tumor cells. J Biol Chem. 1983 Jan 10;258(1):339–343. [PubMed] [Google Scholar]
- Siegfried J. M., Burke T. G., Tritton T. R. Cellular transport of anthracyclines by passive diffusion. Implications for drug resistance. Biochem Pharmacol. 1985 Mar 1;34(5):593–598. doi: 10.1016/0006-2952(85)90251-5. [DOI] [PubMed] [Google Scholar]
- Sircar S., Cai F., Begin M. E., Weber J. M. Transformation renders MDR cells more sensitive to polyunsaturated fatty acids. Anticancer Res. 1990 Nov-Dec;10(6):1783–1786. [PubMed] [Google Scholar]
- Spector A. A., Burns C. P. Biological and therapeutic potential of membrane lipid modification in tumors. Cancer Res. 1987 Sep 1;47(17):4529–4537. [PubMed] [Google Scholar]
- Steel G. G., Peckham M. J. Exploitable mechanisms in combined radiotherapy-chemotherapy: the concept of additivity. Int J Radiat Oncol Biol Phys. 1979 Jan;5(1):85–91. doi: 10.1016/0360-3016(79)90044-0. [DOI] [PubMed] [Google Scholar]
- Steel G. G. Terminology in the description of drug-radiation interactions. Int J Radiat Oncol Biol Phys. 1979 Aug;5(8):1145–1150. doi: 10.1016/0360-3016(79)90634-5. [DOI] [PubMed] [Google Scholar]
- Timmer-Bosscha H., Hospers G. A., Meijer C., Mulder N. H., Muskiet F. A., Martini I. A., Uges D. R., de Vries E. G. Influence of docosahexaenoic acid on cisplatin resistance in a human small cell lung carcinoma cell line. J Natl Cancer Inst. 1989 Jul 19;81(14):1069–1075. doi: 10.1093/jnci/81.14.1069. [DOI] [PubMed] [Google Scholar]
- Tisdale M. J., Beck S. A. Inhibition of tumour-induced lipolysis in vitro and cachexia and tumour growth in vivo by eicosapentaenoic acid. Biochem Pharmacol. 1991 Jan 1;41(1):103–107. doi: 10.1016/0006-2952(91)90016-x. [DOI] [PubMed] [Google Scholar]
- Van der Merwe C. F., Booyens J., Katzeff I. E. Oral gamma-linolenic acid in 21 patients with untreatable malignancy. An ongoing pilot open clinical trial. Br J Clin Pract. 1987 Sep;41(9):907–915. [PubMed] [Google Scholar]
- Wheeler C., Rader R., Kessel D. Membrane alterations associated with progressive adriamycin resistance. Biochem Pharmacol. 1982 Aug 15;31(16):2691–2693. doi: 10.1016/0006-2952(82)90723-7. [DOI] [PubMed] [Google Scholar]
- Wright S., Burton J. L. Oral evening-primrose-seed oil improves atopic eczema. Lancet. 1982 Nov 20;2(8308):1120–1122. doi: 10.1016/s0140-6736(82)92784-2. [DOI] [PubMed] [Google Scholar]
- Zhu Y. P., Su Z. W., Li C. H. Growth-inhibition effects of oleic acid, linoleic acid, and their methyl esters on transplanted tumors in mice. J Natl Cancer Inst. 1989 Sep 6;81(17):1302–1306. doi: 10.1093/jnci/81.17.1302. [DOI] [PubMed] [Google Scholar]
- Zijlstra J. G., de Vries E. G., Muskiet F. A., Martini I. A., Timmer-Bosscha H., Mulder N. H. Influence of docosahexaenoic acid in vitro on intracellular adriamycin concentration in lymphocytes and human adriamycin-sensitive and -resistant small-cell lung cancer cell lines, and on cytotoxicity in the tumor cell lines. Int J Cancer. 1987 Dec 15;40(6):850–856. doi: 10.1002/ijc.2910400625. [DOI] [PubMed] [Google Scholar]


