Abstract
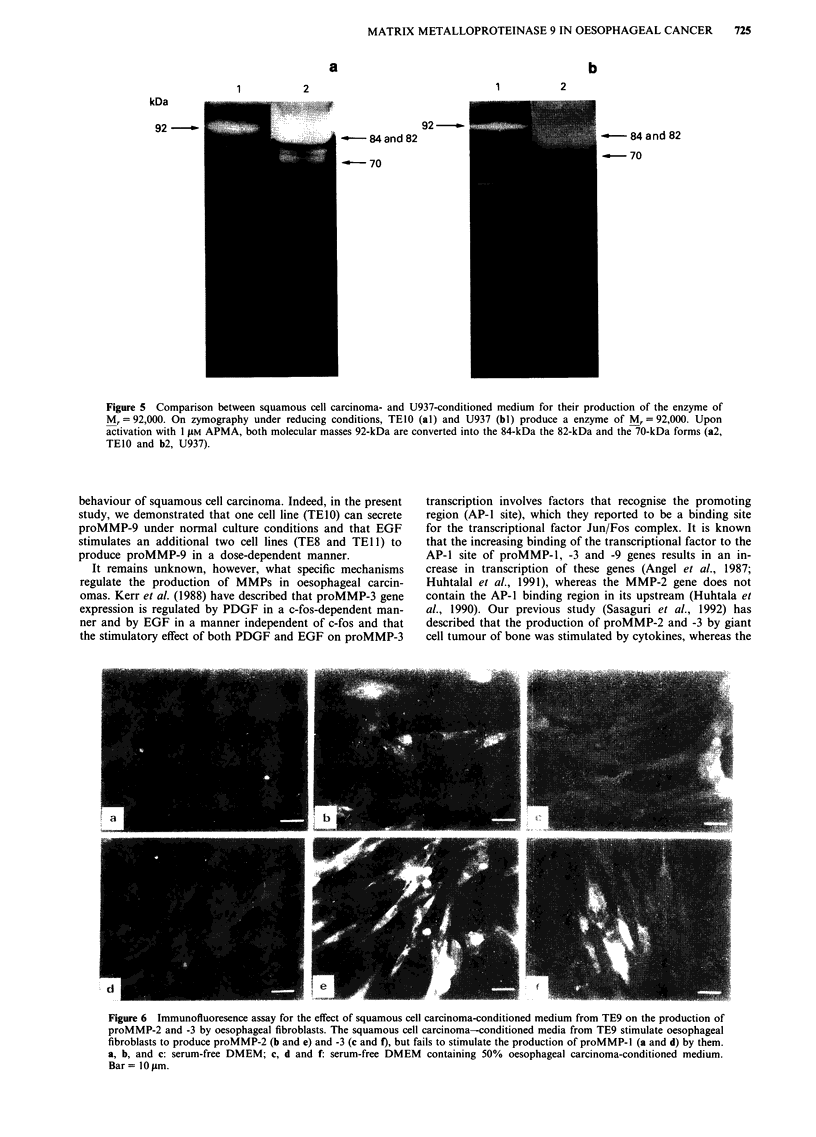
We demonstrated that four human oesophageal squamous cell carcinoma cell lines (TE8, TE9, TE10 and TE11) produced matrix metalloproteinase-1 (proMMP-1/tissue collagenase), 2 (ProMMP-2/'type IV collagenase'), 3 (proMMP-3/stromelysin), and 9 (proMMP-9/92-kDa gelatinase) as members of a matrix metalloproteinase (MMP) family, which degrades extracellular matrix macromolecules. Under normal culture conditions, in immunoblot analysis, proMMP-1 of M(r) = 53,00 was detected in one cell line (TE8), proMMP-2 of M(r) = 72,000 in three cell lines (TE9, TE10, and TE11), and proMMP-3 of M(r) = 57,000 in all four cell lines. In addition to these enzymes, in enzymography, a gelatinolytic activity around M(r) = 92-kDa, likely to be proMMP-9, was detected in only one cell line (TE10) under normal culture conditions. When these cell lines were treated with epidermal growth factor (EGF), however, the agent stimulated three cell lines (TE8, TE10 and TE11) to produce proMMP-9 in a dose-dose dependent manner. Oesophageal carcinoma-conditioned medium stimulated oesophageal fibroblasts to produce proMMP-1, -2, and -3, suggesting that the interaction between oesophageal carcinoma and stromal fibroblasts also plays a role in the production of MMPs by the latter. Our present study illustrates that oesophageal squamous cell carcinoma produces a variety of MMPs including proMMP-1, -2, -3, and -9 in vitro, suggesting that the ability of MMP production of the tumour may play an important role in its malignant behaviour and that the production of proMMP-9 may be regulated by EGF via overexpression of EGF receptors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaishi T. [Several characteristics of tissue cultured human esophageal cell lines]. Nihon Geka Gakkai Zasshi. 1984 Nov;85(11):1440–1453. [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Ballin M., Gomez D. E., Sinha C. C., Thorgeirsson U. P. Ras oncogene mediated induction of a 92 kDa metalloproteinase; strong correlation with the malignant phenotype. Biochem Biophys Res Commun. 1988 Aug 15;154(3):832–838. doi: 10.1016/0006-291x(88)90215-x. [DOI] [PubMed] [Google Scholar]
- Chua C. C., Geiman D. E., Keller G. H., Ladda R. L. Induction of collagenase secretion in human fibroblast cultures by growth promoting factors. J Biol Chem. 1985 May 10;260(9):5213–5216. [PubMed] [Google Scholar]
- Collier I. E., Smith J., Kronberger A., Bauer E. A., Wilhelm S. M., Eisen A. Z., Goldberg G. I. The structure of the human skin fibroblast collagenase gene. J Biol Chem. 1988 Aug 5;263(22):10711–10713. [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Derynck R., Goeddel D. V., Ullrich A., Gutterman J. U., Williams R. D., Bringman T. S., Berger W. H. Synthesis of messenger RNAs for transforming growth factors alpha and beta and the epidermal growth factor receptor by human tumors. Cancer Res. 1987 Feb 1;47(3):707–712. [PubMed] [Google Scholar]
- Garbisa S., Ballin M., Daga-Gordini D., Fastelli G., Naturale M., Negro A., Semenzato G., Liotta L. A. Transient expression of type IV collagenolytic metalloproteinase by human mononuclear phagocytes. J Biol Chem. 1986 Feb 15;261(5):2369–2375. [PubMed] [Google Scholar]
- Goldberg G. I., Frisch S. M., He C., Wilhelm S. M., Reich R., Collier I. E. Secreted proteases. Regulation of their activity and their possible role in metastasis. Ann N Y Acad Sci. 1990;580:375–384. doi: 10.1111/j.1749-6632.1990.tb17945.x. [DOI] [PubMed] [Google Scholar]
- Huhtala P., Chow L. T., Tryggvason K. Structure of the human type IV collagenase gene. J Biol Chem. 1990 Jul 5;265(19):11077–11082. [PubMed] [Google Scholar]
- Huhtala P., Tuuttila A., Chow L. T., Lohi J., Keski-Oja J., Tryggvason K. Complete structure of the human gene for 92-kDa type IV collagenase. Divergent regulation of expression for the 92- and 72-kilodalton enzyme genes in HT-1080 cells. J Biol Chem. 1991 Sep 5;266(25):16485–16490. [PubMed] [Google Scholar]
- Irimura T., Yamori T., Bennett S. C., Ota D. M., Cleary K. R. The relationship of collagenolytic activity to stage of human colorectal carcinoma. Int J Cancer. 1987 Jul 15;40(1):24–31. doi: 10.1002/ijc.2910400106. [DOI] [PubMed] [Google Scholar]
- Kalebic T., Garbisa S., Glaser B., Liotta L. A. Basement membrane collagen: degradation by migrating endothelial cells. Science. 1983 Jul 15;221(4607):281–283. doi: 10.1126/science.6190230. [DOI] [PubMed] [Google Scholar]
- Kamata N., Chida K., Rikimaru K., Horikoshi M., Enomoto S., Kuroki T. Growth-inhibitory effects of epidermal growth factor and overexpression of its receptors on human squamous cell carcinomas in culture. Cancer Res. 1986 Apr;46(4 Pt 1):1648–1653. [PubMed] [Google Scholar]
- Kerr L. D., Holt J. T., Matrisian L. M. Growth factors regulate transin gene expression by c-fos-dependent and c-fos-independent pathways. Science. 1988 Dec 9;242(4884):1424–1427. doi: 10.1126/science.2462278. [DOI] [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986 Jan;46(1):1–7. [PubMed] [Google Scholar]
- Lyons J. G., Birkedal-Hansen B., Moore W. G., O'Grady R. L., Birkedal-Hansen H. Characteristics of a 95-kDa matrix metalloproteinase produced by mammary carcinoma cells. Biochemistry. 1991 Feb 12;30(6):1449–1456. doi: 10.1021/bi00220a001. [DOI] [PubMed] [Google Scholar]
- Monteagudo C., Merino M. J., San-Juan J., Liotta L. A., Stetler-Stevenson W. G. Immunohistochemical distribution of type IV collagenase in normal, benign, and malignant breast tissue. Am J Pathol. 1990 Mar;136(3):585–592. [PMC free article] [PubMed] [Google Scholar]
- Morodomi T., Ogata Y., Sasaguri Y., Morimatsu M., Nagase H. Purification and characterization of matrix metalloproteinase 9 from U937 monocytic leukaemia and HT1080 fibrosarcoma cells. Biochem J. 1992 Jul 15;285(Pt 2):603–611. doi: 10.1042/bj2850603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Hembry R. M., McGarrity A. M., Reynolds J. J., Henderson B. Gelatinase (type IV collagenase) immunolocalization in cells and tissues: use of an antiserum to rabbit bone gelatinase that identifies high and low Mr forms. J Cell Sci. 1989 Mar;92(Pt 3):487–495. doi: 10.1242/jcs.92.3.487. [DOI] [PubMed] [Google Scholar]
- Murphy G., McAlpine C. G., Poll C. T., Reynolds J. J. Purification and characterization of a bone metalloproteinase that degrades gelatin and types IV and V collagen. Biochim Biophys Acta. 1985 Sep 20;831(1):49–58. doi: 10.1016/0167-4838(85)90148-7. [DOI] [PubMed] [Google Scholar]
- Niedbala M. J., Sartorelli A. C. Regulation by epidermal growth factor of human squamous cell carcinoma plasminogen activator-mediated proteolysis of extracellular matrix. Cancer Res. 1989 Jun 15;49(12):3302–3309. [PubMed] [Google Scholar]
- Okada Y., Morodomi T., Enghild J. J., Suzuki K., Yasui A., Nakanishi I., Salvesen G., Nagase H. Matrix metalloproteinase 2 from human rheumatoid synovial fibroblasts. Purification and activation of the precursor and enzymic properties. Eur J Biochem. 1990 Dec 27;194(3):721–730. doi: 10.1111/j.1432-1033.1990.tb19462.x. [DOI] [PubMed] [Google Scholar]
- Okada Y., Nagase H., Harris E. D., Jr A metalloproteinase from human rheumatoid synovial fibroblasts that digests connective tissue matrix components. Purification and characterization. J Biol Chem. 1986 Oct 25;261(30):14245–14255. [PubMed] [Google Scholar]
- Okada Y., Takeuchi N., Tomita K., Nakanishi I., Nagase H. Immunolocalization of matrix metalloproteinase 3 (stromelysin) in rheumatoid synovioblasts (B cells): correlation with rheumatoid arthritis. Ann Rheum Dis. 1989 Aug;48(8):645–653. doi: 10.1136/ard.48.8.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne B., Richards C. S., Hendler F., Burns D., Gusterson B. Over-expression of the EGF receptor is a hallmark of squamous cell carcinomas. J Pathol. 1986 May;149(1):9–14. doi: 10.1002/path.1711490104. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Ueda M., Ando N., Abe O., Shimizu N. High incidence of EGF receptor hyperproduction in esophageal squamous-cell carcinomas. Int J Cancer. 1987 Mar 15;39(3):333–337. doi: 10.1002/ijc.2910390311. [DOI] [PubMed] [Google Scholar]
- Sasaguri Y., Komiya S., Sugama K., Suzuki K., Inoue A., Morimatsu M., Nagase H. Production of matrix metalloproteinases 2 and 3 (stromelysin) by stromal cells of giant cell tumor of bone. Am J Pathol. 1992 Sep;141(3):611–621. [PMC free article] [PubMed] [Google Scholar]
- Sasaguri Y., Yanagi H., Nagase H., Nakano R., Fukuda S., Morimatsu M. Collagenase production by immortalized human aortic endothelial cells infected with simian virus 40. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;60(2):91–97. doi: 10.1007/BF02899532. [DOI] [PubMed] [Google Scholar]
- Shima I., Sasaguri Y., Kusukawa J., Yamana H., Fujita H., Kakegawa T., Morimatsu M. Production of matrix metalloproteinase-2 and metalloproteinase-3 related to malignant behavior of esophageal carcinoma. A clinicopathologic study. Cancer. 1992 Dec 15;70(12):2747–2753. doi: 10.1002/1097-0142(19921215)70:12<2747::aid-cncr2820701204>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Sloane B. F., Honn K. V. Cysteine proteinases and metastasis. Cancer Metastasis Rev. 1984;3(3):249–263. doi: 10.1007/BF00048388. [DOI] [PubMed] [Google Scholar]
- Uitto V. J., Schwartz D., Veis A. Degradation of basement-membrane collagen by neutral proteases from human leukocytes. Eur J Biochem. 1980 Apr;105(2):409–417. doi: 10.1111/j.1432-1033.1980.tb04515.x. [DOI] [PubMed] [Google Scholar]
- Welgus H. G., Jeffrey J. J., Eisen A. Z. The collagen substrate specificity of human skin fibroblast collagenase. J Biol Chem. 1981 Sep 25;256(18):9511–9515. [PubMed] [Google Scholar]
- Wilhelm S. M., Collier I. E., Marmer B. L., Eisen A. Z., Grant G. A., Goldberg G. I. SV40-transformed human lung fibroblasts secrete a 92-kDa type IV collagenase which is identical to that secreted by normal human macrophages. J Biol Chem. 1989 Oct 15;264(29):17213–17221. [PubMed] [Google Scholar]
- Yamagata S., Tanaka R., Ito Y., Shimizu S. Gelatinases of murine metastatic tumor cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):228–234. doi: 10.1016/s0006-291x(89)80202-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Kamata N., Kawano H., Shimizu S., Kuroki T., Toyoshima K., Rikimaru K., Nomura N., Ishizaki R., Pastan I. High incidence of amplification of the epidermal growth factor receptor gene in human squamous carcinoma cell lines. Cancer Res. 1986 Jan;46(1):414–416. [PubMed] [Google Scholar]
- Yanagi H., Sasaguri Y., Sugama K., Morimatsu M., Nagase H. Production of tissue collagenase (matrix metalloproteinase 1) by human aortic smooth muscle cells in response to platelet-derived growth factor. Atherosclerosis. 1991 Dec;91(3):207–216. doi: 10.1016/0021-9150(91)90168-3. [DOI] [PubMed] [Google Scholar]
- Yano H., Shiozaki H., Kobayashi K., Yano T., Tahara H., Tamura S., Mori T. Immunohistologic detection of the epidermal growth factor receptor in human esophageal squamous cell carcinoma. Cancer. 1991 Jan 1;67(1):91–98. doi: 10.1002/1097-0142(19910101)67:1<91::aid-cncr2820670118>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]