Abstract
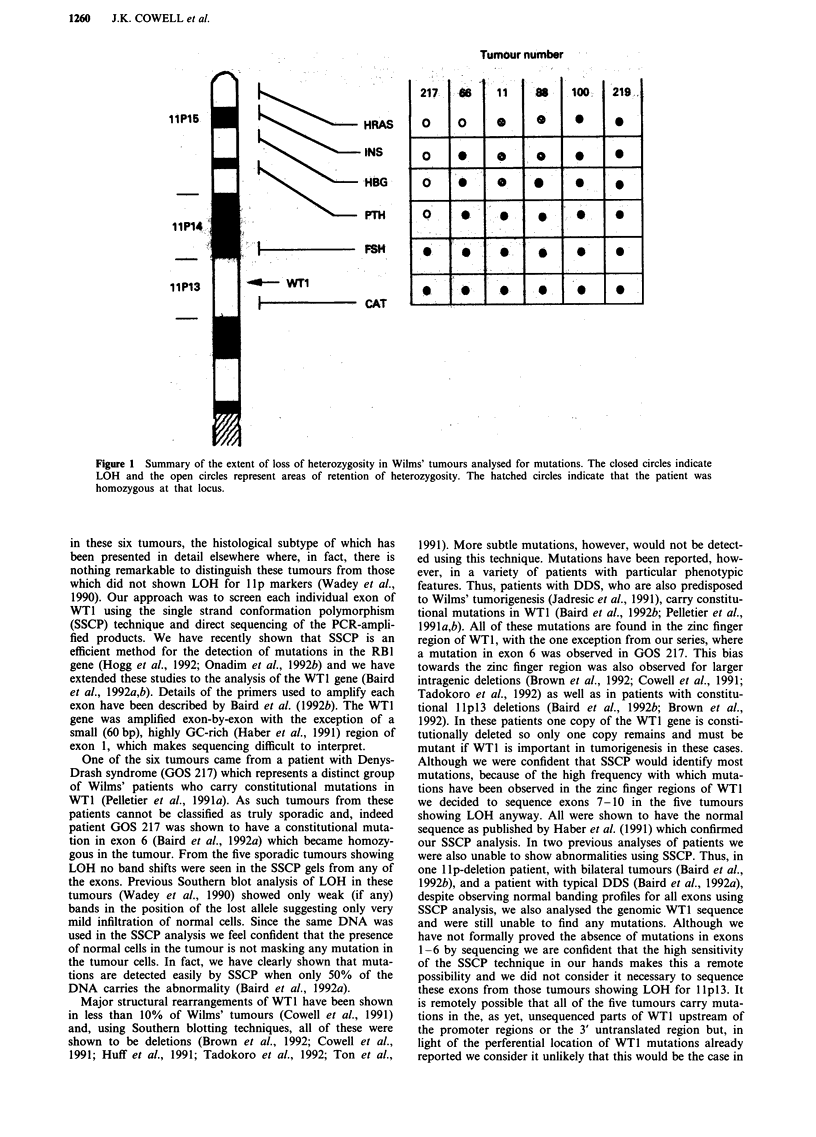
Loss of heterozygosity (LOH) in tumour cells is generally accepted as 'exposing' recessive cancer genes. The short arm of chromosome 11 shows consistent LOH in Wilms' tumours along its entire length. Occasionally, however, only the 11p13 and/or the 11p15 regions are involved. Deletions of the 11p13 region consistently predisposes to Wilms' tumorigenesis. We have analysed the recently cloned WT1 gene from the 11p13 region exon-by-exon in five tumours previously shown to have undergone LOH for the 11p13 region, using single strand conformation polymorphism analysis (SSCP) and PCR sequencing. Our analysis using SSCP failed to identify any band shifts in the WT1 gene from these tumours. In addition we also sequenced the zinc finger region of WT1, which is the part of the gene most frequently showing mutations. Only the normal sequence was found in all of these tumours. These results demonstrate that LOH in Wilms' tumours is not always related to mutations in the WT1 genes and argues strongly that another gene, probably in the 11p15 region, may be more important in Wilms' tumorigenesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird P. N., Groves N., Haber D. A., Housman D. E., Cowell J. K. Identification of mutations in the WT1 gene in tumours from patients with the WAGR syndrome. Oncogene. 1992 Nov;7(11):2141–2149. [PubMed] [Google Scholar]
- Baird P. N., Santos A., Groves N., Jadresic L., Cowell J. K. Constitutional mutations in the WT1 gene in patients with Denys-Drash syndrome. Hum Mol Genet. 1992 Aug;1(5):301–305. doi: 10.1093/hmg/1.5.301. [DOI] [PubMed] [Google Scholar]
- Brown K. W., Watson J. E., Poirier V., Mott M. G., Berry P. J., Maitland N. J. Inactivation of the remaining allele of the WT1 gene in a Wilms' tumour from a WAGR patient. Oncogene. 1992 Apr;7(4):763–768. [PubMed] [Google Scholar]
- Call K. M., Glaser T., Ito C. Y., Buckler A. J., Pelletier J., Haber D. A., Rose E. A., Kral A., Yeger H., Lewis W. H. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms' tumor locus. Cell. 1990 Feb 9;60(3):509–520. doi: 10.1016/0092-8674(90)90601-a. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Comings D. E. A general theory of carcinogenesis. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3324–3328. doi: 10.1073/pnas.70.12.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell J. K., Wadey R. B., Haber D. A., Call K. M., Housman D. E., Pritchard J. Structural rearrangements of the WT1 gene in Wilms' tumour cells. Oncogene. 1991 Apr;6(4):595–599. [PubMed] [Google Scholar]
- Dunn J. M., Phillips R. A., Zhu X., Becker A., Gallie B. L. Mutations in the RB1 gene and their effects on transcription. Mol Cell Biol. 1989 Nov;9(11):4596–4604. doi: 10.1128/mcb.9.11.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessler M., Poustka A., Cavenee W., Neve R. L., Orkin S. H., Bruns G. A. Homozygous deletion in Wilms tumours of a zinc-finger gene identified by chromosome jumping. Nature. 1990 Feb 22;343(6260):774–778. doi: 10.1038/343774a0. [DOI] [PubMed] [Google Scholar]
- Haber D. A., Sohn R. L., Buckler A. J., Pelletier J., Call K. M., Housman D. E. Alternative splicing and genomic structure of the Wilms tumor gene WT1. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9618–9622. doi: 10.1073/pnas.88.21.9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg A., Onadim Z., Baird P. N., Cowell J. K. Detection of heterozygous mutations in the RB1 gene in retinoblastoma patients using single-strand conformation polymorphism analysis and polymerase chain reaction sequencing. Oncogene. 1992 Jul;7(7):1445–1451. [PubMed] [Google Scholar]
- Huff V., Miwa H., Haber D. A., Call K. M., Housman D., Strong L. C., Saunders G. F. Evidence for WT1 as a Wilms tumor (WT) gene: intragenic germinal deletion in bilateral WT. Am J Hum Genet. 1991 May;48(5):997–1003. [PMC free article] [PubMed] [Google Scholar]
- Jadresic L., Wadey R. B., Buckle B., Barratt T. M., Mitchell C. D., Cowell J. K. Molecular analysis of chromosome region 11p13 in patients with Drash syndrome. Hum Genet. 1991 Mar;86(5):497–501. doi: 10.1007/BF00194641. [DOI] [PubMed] [Google Scholar]
- Mannens M., Slater R. M., Heyting C., Bliek J., de Kraker J., Coad N., de Pagter-Holthuizen P., Pearson P. L. Molecular nature of genetic changes resulting in loss of heterozygosity of chromosome 11 in Wilms' tumours. Hum Genet. 1988 Dec;81(1):41–48. doi: 10.1007/BF00283727. [DOI] [PubMed] [Google Scholar]
- Narahara K., Kikkawa K., Kimira S., Kimoto H., Ogata M., Kasai R., Hamawaki M., Matsuoka K. Regional mapping of catalase and Wilms tumor--aniridia, genitourinary abnormalities, and mental retardation triad loci to the chromosome segment 11p1305----p1306. Hum Genet. 1984;66(2-3):181–185. doi: 10.1007/BF00286597. [DOI] [PubMed] [Google Scholar]
- Onadim Z., Hogg A., Baird P. N., Cowell J. K. Oncogenic point mutations in exon 20 of the RB1 gene in families showing incomplete penetrance and mild expression of the retinoblastoma phenotype. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6177–6181. doi: 10.1073/pnas.89.13.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Bruening W., Kashtan C. E., Mauer S. M., Manivel J. C., Striegel J. E., Houghton D. C., Junien C., Habib R., Fouser L. Germline mutations in the Wilms' tumor suppressor gene are associated with abnormal urogenital development in Denys-Drash syndrome. Cell. 1991 Oct 18;67(2):437–447. doi: 10.1016/0092-8674(91)90194-4. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Bruening W., Li F. P., Haber D. A., Glaser T., Housman D. E. WT1 mutations contribute to abnormal genital system development and hereditary Wilms' tumour. Nature. 1991 Oct 3;353(6343):431–434. doi: 10.1038/353431a0. [DOI] [PubMed] [Google Scholar]
- Pritchard-Jones K., Fleming S. Cell types expressing the Wilms' tumour gene (WT1) in Wilms' tumours: implications for tumour histogenesis. Oncogene. 1991 Dec;6(12):2211–2220. [PubMed] [Google Scholar]
- Riccardi V. M., Sujansky E., Smith A. C., Francke U. Chromosomal imbalance in the Aniridia-Wilms' tumor association: 11p interstitial deletion. Pediatrics. 1978 Apr;61(4):604–610. [PubMed] [Google Scholar]
- Stanbridge E. J. Human tumor suppressor genes. Annu Rev Genet. 1990;24:615–657. doi: 10.1146/annurev.ge.24.120190.003151. [DOI] [PubMed] [Google Scholar]
- Tadokoro K., Fujii H., Ohshima A., Kakizawa Y., Shimizu K., Sakai A., Sumiyoshi K., Inoue T., Hayashi Y., Yamada M. Intragenic homozygous deletion of the WT1 gene in Wilms' tumor. Oncogene. 1992 Jun;7(6):1215–1221. [PubMed] [Google Scholar]
- Ton C. C., Huff V., Call K. M., Cohn S., Strong L. C., Housman D. E., Saunders G. F. Smallest region of overlap in Wilms tumor deletions uniquely implicates an 11p13 zinc finger gene as the disease locus. Genomics. 1991 May;10(1):293–297. doi: 10.1016/0888-7543(91)90516-h. [DOI] [PubMed] [Google Scholar]
- Wadey R. B., Pal N., Buckle B., Yeomans E., Pritchard J., Cowell J. K. Loss of heterozygosity in Wilms' tumour involves two distinct regions of chromosome 11. Oncogene. 1990 Jun;5(6):901–907. [PubMed] [Google Scholar]
- Waziri M., Patil S. R., Hanson J. W., Bartley J. A. Abnormality of chromosome 11 in patients with features of Beckwith-Wiedemann syndrome. J Pediatr. 1983 Jun;102(6):873–876. doi: 10.1016/s0022-3476(83)80014-6. [DOI] [PubMed] [Google Scholar]


