Abstract
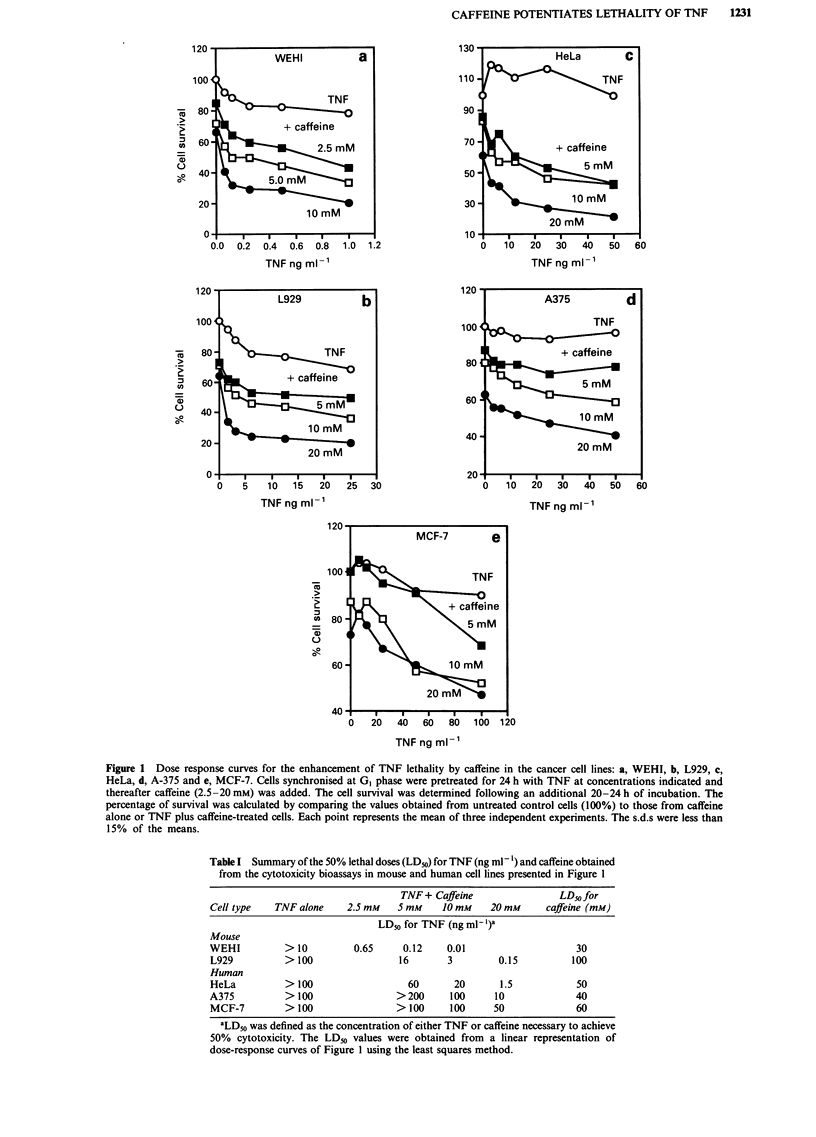
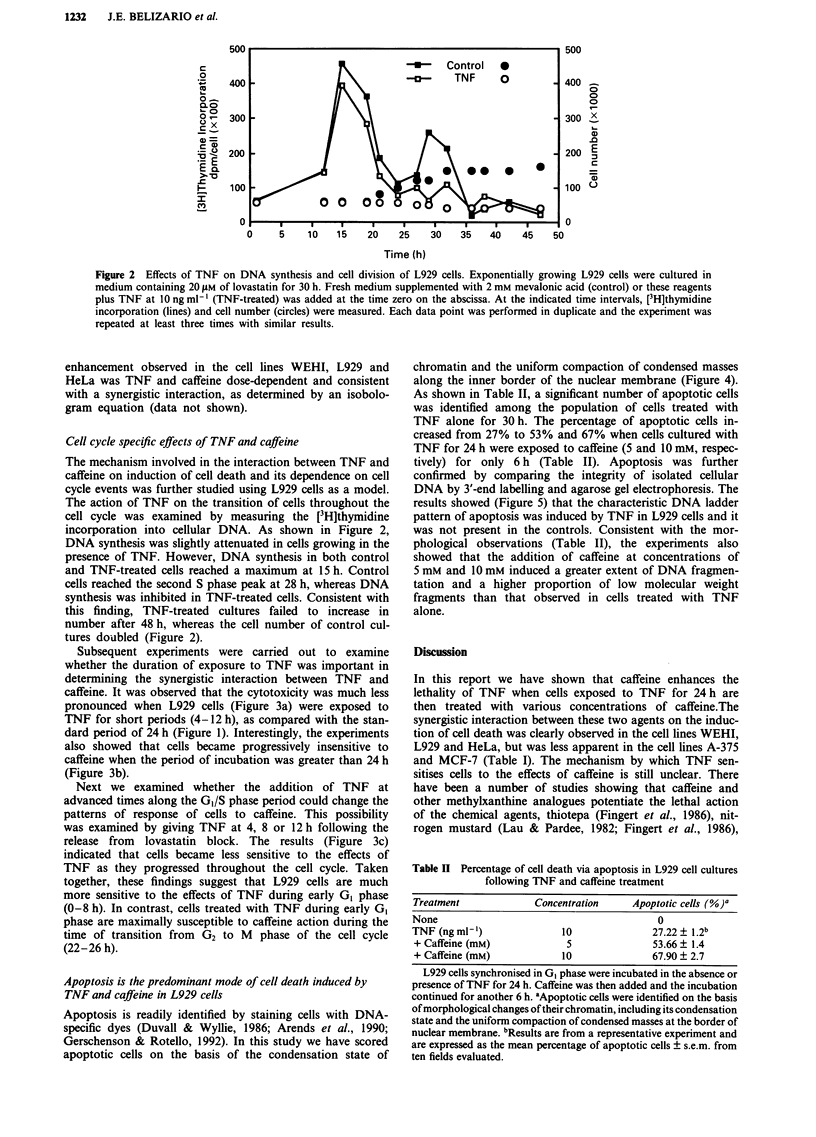
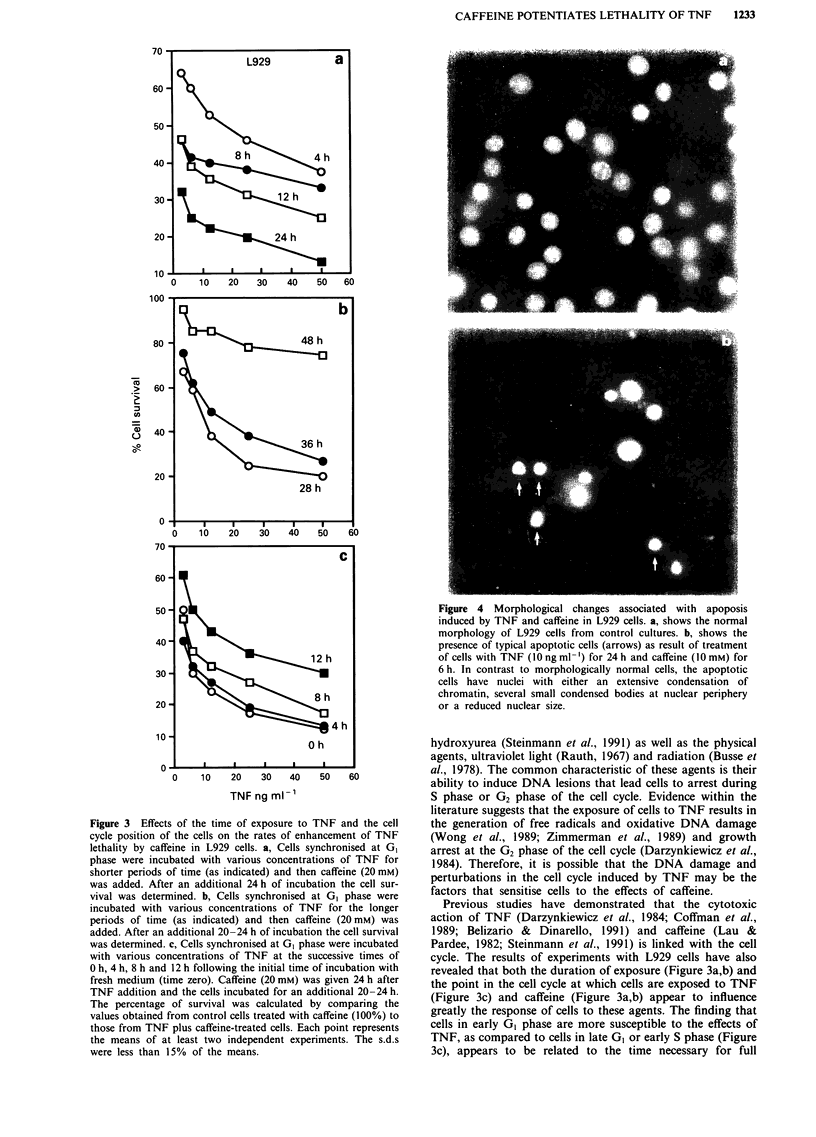
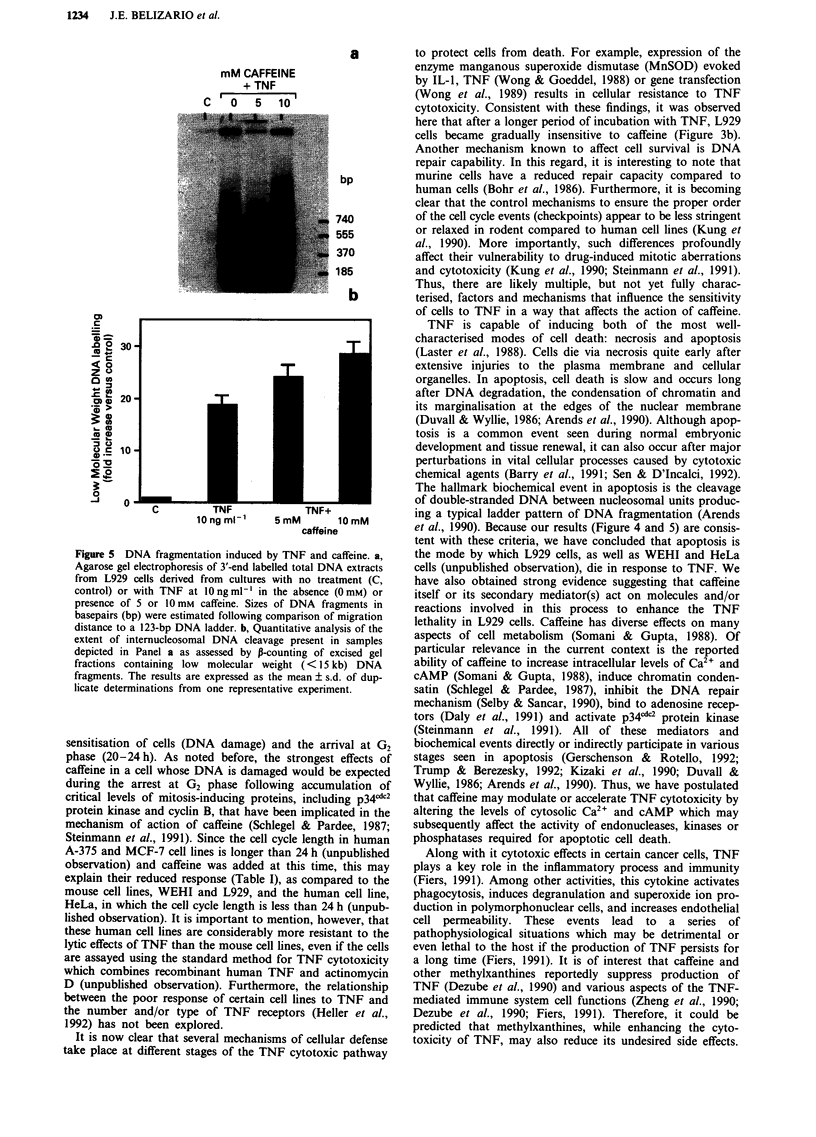
In this study we have investigated the interaction of caffeine, a prototypic methylxanthine, and TNF on the induction of cell death in mouse and human cell lines during progression from G1 to successive phases of the cell cycle. Exposure of cells to TNF (0.1-100 ng ml-1) as single agent for 48 h caused low or no lethality. The rates of cell death increased significantly when cells cultured with TNF for 24 h were exposed to caffeine (2.5-20 mM). The magnitude of the enhancement by caffeine was TNF and caffeine dose-dependent. The most effective response to this combination was observed in the mouse cell lines, WEHI and L929, followed by the human cell lines, HeLa, A375 and MCF-7, respectively. In L929 cells, TNF treatment did not inhibit DNA synthesis during the first S phase of the cell cycle (20-24 h), but it did block the progress toward a second S phase, indicating the cells were arrested at G2 phase or mitosis. Caffeine had great enhancer effect on L929 cells exposed to TNF for 24 h, but the effect was reduced in cells with either less than 24 h or greater than 28 h of exposure. L929 cells stimulated with TNF died via apoptosis, as judged by both morphological criteria and the occurrence of internucleosomal DNA cleavage. Exposure of TNF-treated cells to caffeine caused a greater increase in the proportion of apoptotic cells as well as the extent of internucleosomal DNA fragmentation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal S., Drysdale B. E., Shin H. S. Tumor necrosis factor-mediated cytotoxicity involves ADP-ribosylation. J Immunol. 1988 Jun 15;140(12):4187–4192. [PubMed] [Google Scholar]
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Baloch Z., Cohen S., Coffman F. D. Synergistic interactions between tumor necrosis factor and inhibitors of DNA topoisomerase I and II. J Immunol. 1990 Nov 1;145(9):2908–2913. [PubMed] [Google Scholar]
- Barry M. A., Behnke C. A., Eastman A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem Pharmacol. 1990 Nov 15;40(10):2353–2362. doi: 10.1016/0006-2952(90)90733-2. [DOI] [PubMed] [Google Scholar]
- Belizario J. E., Dinarello C. A. Interleukin 1, interleukin 6, tumor necrosis factor, and transforming growth factor beta increase cell resistance to tumor necrosis factor cytotoxicity by growth arrest in the G1 phase of the cell cycle. Cancer Res. 1991 May 1;51(9):2379–2385. [PubMed] [Google Scholar]
- Bohr V. A., Okumoto D. S., Hanawalt P. C. Survival of UV-irradiated mammalian cells correlates with efficient DNA repair in an essential gene. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3830–3833. doi: 10.1073/pnas.83.11.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse P. M., Bose S. K., Jones R. W., Tolmach L. J. The action of caffeine on X-irradiated HeLa cells. III. Enhancement of X-ray-induced killing during G2 arrest. Radiat Res. 1978 Nov;76(2):292–307. [PubMed] [Google Scholar]
- Coffman F. D., Haviland D. L., Green L. M., Ware C. F. Cytotoxicity by tumor necrosis factor is linked with the cell cycle but does not require DNA synthesis. Growth Factors. 1989;1(4):357–364. doi: 10.3109/08977198909000259. [DOI] [PubMed] [Google Scholar]
- Daly J. W., Hide I., Müller C. E., Shamim M. Caffeine analogs: structure-activity relationships at adenosine receptors. Pharmacology. 1991;42(6):309–321. doi: 10.1159/000138813. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Williamson B., Carswell E. A., Old L. J. Cell cycle-specific effects of tumor necrosis factor. Cancer Res. 1984 Jan;44(1):83–90. [PubMed] [Google Scholar]
- Dezube B. J., Eder J. P., Pardee A. B. Phase I trial of escalating pentoxifylline dose with constant dose thiotepa. Cancer Res. 1990 Nov 1;50(21):6806–6810. [PubMed] [Google Scholar]
- Fiers W. Tumor necrosis factor. Characterization at the molecular, cellular and in vivo level. FEBS Lett. 1991 Jul 22;285(2):199–212. doi: 10.1016/0014-5793(91)80803-b. [DOI] [PubMed] [Google Scholar]
- Fingert H. J., Chang J. D., Pardee A. B. Cytotoxic, cell cycle, and chromosomal effects of methylxanthines in human tumor cells treated with alkylating agents. Cancer Res. 1986 May;46(5):2463–2467. [PubMed] [Google Scholar]
- Gerschenson L. E., Rotello R. J. Apoptosis: a different type of cell death. FASEB J. 1992 Apr;6(7):2450–2455. doi: 10.1096/fasebj.6.7.1563596. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Weinert T. A. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989 Nov 3;246(4930):629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Heller R. A., Song K., Fan N., Chang D. J. The p70 tumor necrosis factor receptor mediates cytotoxicity. Cell. 1992 Jul 10;70(1):47–56. doi: 10.1016/0092-8674(92)90532-h. [DOI] [PubMed] [Google Scholar]
- Jakóbisiak M., Bruno S., Skierski J. S., Darzynkiewicz Z. Cell cycle-specific effects of lovastatin. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3628–3632. doi: 10.1073/pnas.88.9.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyomarsi K., Sandoval L., Band V., Pardee A. B. Synchronization of tumor and normal cells from G1 to multiple cell cycles by lovastatin. Cancer Res. 1991 Jul 1;51(13):3602–3609. [PubMed] [Google Scholar]
- Kizaki H., Suzuki K., Tadakuma T., Ishimura Y. Adenosine receptor-mediated accumulation of cyclic AMP-induced T-lymphocyte death through internucleosomal DNA cleavage. J Biol Chem. 1990 Mar 25;265(9):5280–5284. [PubMed] [Google Scholar]
- Kung A. L., Sherwood S. W., Schimke R. T. Cell line-specific differences in the control of cell cycle progression in the absence of mitosis. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9553–9557. doi: 10.1073/pnas.87.24.9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laster S. M., Wood J. G., Gooding L. R. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol. 1988 Oct 15;141(8):2629–2634. [PubMed] [Google Scholar]
- Lau C. C., Pardee A. B. Mechanism by which caffeine potentiates lethality of nitrogen mustard. Proc Natl Acad Sci U S A. 1982 May;79(9):2942–2946. doi: 10.1073/pnas.79.9.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B. G1 events and regulation of cell proliferation. Science. 1989 Nov 3;246(4930):603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Rauth A. M. Evidence for dark-reactivation of ultraviolet light damage in mouse L cells. Radiat Res. 1967 May;31(1):121–138. [PubMed] [Google Scholar]
- Schlegel R., Pardee A. B. Periodic mitotic events induced in the absence of DNA replication. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9025–9029. doi: 10.1073/pnas.84.24.9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby C. P., Sancar A. Molecular mechanisms of DNA repair inhibition by caffeine. Proc Natl Acad Sci U S A. 1990 May;87(9):3522–3525. doi: 10.1073/pnas.87.9.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S., D'Incalci M. Apoptosis. Biochemical events and relevance to cancer chemotherapy. FEBS Lett. 1992 Jul 27;307(1):122–127. doi: 10.1016/0014-5793(92)80914-3. [DOI] [PubMed] [Google Scholar]
- Somani S. M., Gupta P. Caffeine: a new look at an age-old drug. Int J Clin Pharmacol Ther Toxicol. 1988 Nov;26(11):521–533. [PubMed] [Google Scholar]
- Steinmann K. E., Belinsky G. S., Lee D., Schlegel R. Chemically induced premature mitosis: differential response in rodent and human cells and the relationship to cyclin B synthesis and p34cdc2/cyclin B complex formation. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6843–6847. doi: 10.1073/pnas.88.15.6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suffys P., Beyaert R., Van Roy F., Fiers W. Involvement of a serine protease in tumour-necrosis-factor-mediated cytotoxicity. Eur J Biochem. 1988 Dec 1;178(1):257–265. doi: 10.1111/j.1432-1033.1988.tb14451.x. [DOI] [PubMed] [Google Scholar]
- Tilly J. L., Hsueh A. J. Microscale autoradiographic method for the qualitative and quantitative analysis of apoptotic DNA fragmentation. J Cell Physiol. 1993 Mar;154(3):519–526. doi: 10.1002/jcp.1041540310. [DOI] [PubMed] [Google Scholar]
- Tilly J. L., Kowalski K. I., Johnson A. L., Hsueh A. J. Involvement of apoptosis in ovarian follicular atresia and postovulatory regression. Endocrinology. 1991 Nov;129(5):2799–2801. doi: 10.1210/endo-129-5-2799. [DOI] [PubMed] [Google Scholar]
- Trump B. F., Berezesky I. K. The role of cytosolic Ca2+ in cell injury, necrosis and apoptosis. Curr Opin Cell Biol. 1992 Apr;4(2):227–232. doi: 10.1016/0955-0674(92)90037-d. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Elwell J. H., Oberley L. W., Goeddel D. V. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell. 1989 Sep 8;58(5):923–931. doi: 10.1016/0092-8674(89)90944-6. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Induction of manganous superoxide dismutase by tumor necrosis factor: possible protective mechanism. Science. 1988 Nov 11;242(4880):941–944. doi: 10.1126/science.3263703. [DOI] [PubMed] [Google Scholar]
- Zheng H., Crowley J. J., Chan J. C., Hoffmann H., Hatherill J. R., Ishizaka A., Raffin T. A. Attenuation of tumor necrosis factor-induced endothelial cell cytotoxicity and neutrophil chemiluminescence. Am Rev Respir Dis. 1990 Nov;142(5):1073–1078. doi: 10.1164/ajrccm/142.5.1073. [DOI] [PubMed] [Google Scholar]
- Zimmerman R. J., Chan A., Leadon S. A. Oxidative damage in murine tumor cells treated in vitro by recombinant human tumor necrosis factor. Cancer Res. 1989 Apr 1;49(7):1644–1648. [PubMed] [Google Scholar]