Abstract
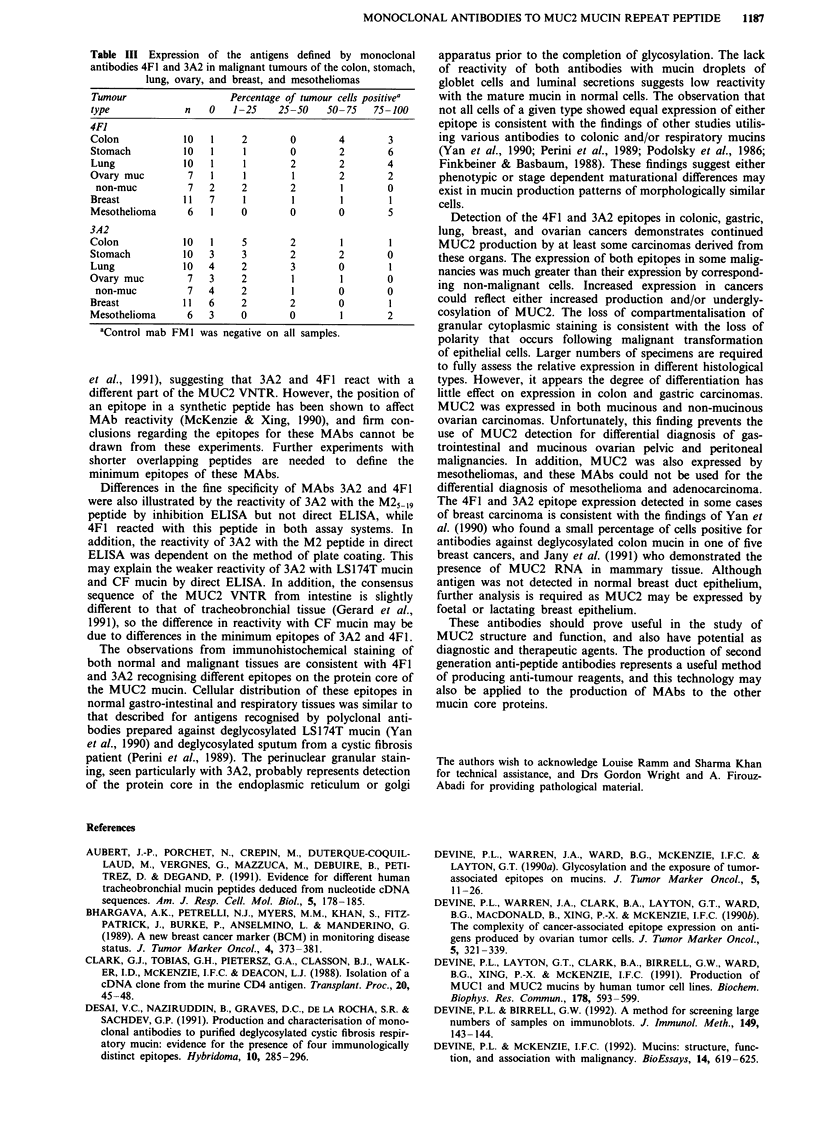
This study sought to produce monoclonal antibodies (MAbs) which reacted with the MUC2 core protein. Two MAbs [3A2 (IgG1) and 4F1 (IgM)] were produced by immunising female BALB/c mice with gel-formed mucin from the LS174T colon cancer cell line followed by a KLH conjugate of a 29 amino acid synthetic peptide whose sequence was derived from the variable number of tandem repeats (VNTR) region of a MUC2 cDNA clone. The MAbs reacted with synthetic MUC2 VNTR peptides but not synthetic MUC1 or MUC3 VNTR peptides, and showed specific reactivity in Western blotting with a high molecular weight protein produced by the LS174T colon carcinoma cell line. The use of shorter peptides indicated that the minimum peptide epitopes for these MAbs were different. Mab 3A2 reacted with amino acids 5-19 of the MUC2 VNTR by inhibition ELISA but not by direct ELISA, while 4F1 reacted with this peptide in both assays. Furthermore, 4F1 reacted in direct ELISA when a larger (29 amino acid) MUC2-derived peptide was coated onto the assay plate by incubating in carbonate buffer or by drying the peptide onto the assay plate, while 3A2 only reacted when this peptide was coated in carbonate buffer. The different specificity of the MAbs was also illustrated by the reactivity of 4F1 but not 3A2 with partially deglycosylated cystic fibrosis mucin. Immunohistochemical analysis with these MAbs revealed a strong reactivity with lung, gastric and colon tumours relative to normal tissue, with some breast and ovarian tumours also reacting. Both MAbs stained some normal goblet cells in the perinuclear region but not the mucin droplet or secreted mucin, indicating a reaction with immature (poorly glycosylated) mucin in the endoplasmic reticulum and/or golgi, but not with mature (fully glycosylated) mucin. In contrast, tumours showed strong diffuse cytoplasmic staining. 4F1 also showed weak apical cytoplasmic staining in some goblet cells and stained some tumours which showed no reactivity with 3A2. These antibodies should prove useful in the study of MUC2 structure and function, and in the diagnosis of some tumours.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubert J. P., Porchet N., Crepin M., Duterque-Coquillaud M., Vergnes G., Mazzuca M., Debuire B., Petitprez D., Degand P. Evidence for different human tracheobronchial mucin peptides deduced from nucleotide cDNA sequences. Am J Respir Cell Mol Biol. 1991 Aug;5(2):178–185. doi: 10.1165/ajrcmb/5.2.178. [DOI] [PubMed] [Google Scholar]
- Clark G. J., Tobias G. H., Pietersz G. A., Classon B. J., Walker I. D., McKenzie I. F., Deacon N. J. Isolation of a cDNA clone for the murine CD4 antigen. Transplant Proc. 1988 Feb;20(1):45–48. [PubMed] [Google Scholar]
- Desai V. C., Naziruddin B., Graves D. C., Reyes de la Rocha S., Sachdev G. P. Production and characterization of monoclonal antibodies to purified deglycosylated cystic fibrosis respiratory mucin: evidence for the presence of four immunologically distinct epitopes. Hybridoma. 1991 Apr;10(2):285–296. doi: 10.1089/hyb.1991.10.285. [DOI] [PubMed] [Google Scholar]
- Devine P. L., Birrell G. W. A method for screening large numbers of samples on immunoblots. J Immunol Methods. 1992 Apr 27;149(1):143–144. doi: 10.1016/s0022-1759(12)80061-5. [DOI] [PubMed] [Google Scholar]
- Devine P. L., Layton G. T., Clark B. A., Birrell G. W., Ward B. G., Xing P. X., McKenzie I. F. Production of MUC1 and MUC2 mucins by human tumor cell lines. Biochem Biophys Res Commun. 1991 Jul 31;178(2):593–599. doi: 10.1016/0006-291x(91)90149-2. [DOI] [PubMed] [Google Scholar]
- Devine P. L., McKenzie I. F. Mucins: structure, function, and associations with malignancy. Bioessays. 1992 Sep;14(9):619–625. doi: 10.1002/bies.950140909. [DOI] [PubMed] [Google Scholar]
- Finkbeiner W. E., Basbaum C. B. Monoclonal antibodies directed against human airway secretions. Localization and characterization of antigens. Am J Pathol. 1988 May;131(2):290–297. [PMC free article] [PubMed] [Google Scholar]
- Gendler S. J., Burchell J. M., Duhig T., Lamport D., White R., Parker M., Taylor-Papadimitriou J. Cloning of partial cDNA encoding differentiation and tumor-associated mucin glycoproteins expressed by human mammary epithelium. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6060–6064. doi: 10.1073/pnas.84.17.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendler S., Taylor-Papadimitriou J., Duhig T., Rothbard J., Burchell J. A highly immunogenic region of a human polymorphic epithelial mucin expressed by carcinomas is made up of tandem repeats. J Biol Chem. 1988 Sep 15;263(26):12820–12823. [PubMed] [Google Scholar]
- Gerard C., Eddy R. L., Jr, Shows T. B. The core polypeptide of cystic fibrosis tracheal mucin contains a tandem repeat structure. Evidence for a common mucin in airway and gastrointestinal tissue. J Clin Invest. 1990 Dec;86(6):1921–1927. doi: 10.1172/JCI114925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geysen H. M., Meloen R. H., Barteling S. J. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3998–4002. doi: 10.1073/pnas.81.13.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gum J. R., Byrd J. C., Hicks J. W., Toribara N. W., Lamport D. T., Kim Y. S. Molecular cloning of human intestinal mucin cDNAs. Sequence analysis and evidence for genetic polymorphism. J Biol Chem. 1989 Apr 15;264(11):6480–6487. [PubMed] [Google Scholar]
- Gum J. R., Hicks J. W., Swallow D. M., Lagace R. L., Byrd J. C., Lamport D. T., Siddiki B., Kim Y. S. Molecular cloning of cDNAs derived from a novel human intestinal mucin gene. Biochem Biophys Res Commun. 1990 Aug 31;171(1):407–415. doi: 10.1016/0006-291x(90)91408-k. [DOI] [PubMed] [Google Scholar]
- Hodges R. S., Merrifield R. B. Monitoring of solid phase peptide synthesis by an automated spectrophotometric picrate method. Anal Biochem. 1975 May 12;65(1-2):241–272. doi: 10.1016/0003-2697(75)90509-6. [DOI] [PubMed] [Google Scholar]
- Houghten R. A. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jany B. H., Gallup M. W., Yan P. S., Gum J. R., Kim Y. S., Basbaum C. B. Human bronchus and intestine express the same mucin gene. J Clin Invest. 1991 Jan;87(1):77–82. doi: 10.1172/JCI115004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layton G. T., Devine P. L., Warren J. A., Birrell G., Xing P. X., Ward B. G., McKenzie I. F. Monoclonal antibodies reactive with the breast carcinoma-associated mucin core protein repeat sequence peptide also recognise the ovarian carcinoma-associated sebaceous gland antigen. Tumour Biol. 1990;11(5):274–286. doi: 10.1159/000217661. [DOI] [PubMed] [Google Scholar]
- Layton G. T., Stanworth D. R., Amos H. E. The specificity of murine polyclonal and monoclonal antibodies to the haptenic drug chlorhexidine induced by chlorine-generated chlorhexidine-protein conjugates. Clin Exp Immunol. 1987 Jul;69(1):157–165. [PMC free article] [PubMed] [Google Scholar]
- McKenzie I. F., Xing P. X. Mucins in breast cancer: recent immunological advances. Cancer Cells. 1990 Mar;2(3):75–78. [PubMed] [Google Scholar]
- Perini J. M., Marianne T., Lafitte J. J., Lamblin G., Roussel P., Mazzuca M. Use of an antiserum against deglycosylated human mucins for cellular localization of their peptide precursors: antigenic similarities between bronchial and intestinal mucins. J Histochem Cytochem. 1989 Jun;37(6):869–875. doi: 10.1177/37.6.2470810. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Fournier D. A., Lynch K. E. Human colonic goblet cells. Demonstration of distinct subpopulations defined by mucin-specific monoclonal antibodies. J Clin Invest. 1986 Apr;77(4):1263–1271. doi: 10.1172/JCI112429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porchet N., Nguyen V. C., Dufosse J., Audie J. P., Guyonnet-Duperat V., Gross M. S., Denis C., Degand P., Bernheim A., Aubert J. P. Molecular cloning and chromosomal localization of a novel human tracheo-bronchial mucin cDNA containing tandemly repeated sequences of 48 base pairs. Biochem Biophys Res Commun. 1991 Mar 15;175(2):414–422. doi: 10.1016/0006-291x(91)91580-6. [DOI] [PubMed] [Google Scholar]
- Price M. R., Pugh J. A., Hudecz F., Griffiths W., Jacobs E., Symonds I. M., Clarke A. J., Chan W. C., Baldwin R. W. C595--a monoclonal antibody against the protein core of human urinary epithelial mucin commonly expressed in breast carcinomas. Br J Cancer. 1990 May;61(5):681–686. doi: 10.1038/bjc.1990.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M. R., Sekowski M., Yang G. Y., Durrant L. G., Robins R. A., Baldwin R. W. Reactivity of an anti-(human gastric carcinoma) monoclonal antibody with core-related peptides of gastrointestinal mucin. Cancer Immunol Immunother. 1991;33(2):80–84. doi: 10.1007/BF01742533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safi F., Kohler I., Röttinger E., Beger H. The value of the tumor marker CA 15-3 in diagnosing and monitoring breast cancer. A comparative study with carcinoembryonic antigen. Cancer. 1991 Aug 1;68(3):574–582. doi: 10.1002/1097-0142(19910801)68:3<574::aid-cncr2820680322>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Shibier O., Hampton S. M., Marks V. An immunisation protocol which enhances the frequency of antigen-specific monoclonal antibody production. J Immunol Methods. 1988 Nov 10;114(1-2):49–52. doi: 10.1016/0022-1759(88)90152-4. [DOI] [PubMed] [Google Scholar]
- Ward B. G., McGuckin M. A., Ramm L. E., Coglan M., Sanderson B., Tripcony L., Free K. E. The management of ovarian carcinoma is improved by the use of cancer-associated serum antigen and CA 125 assays. Cancer. 1993 Jan 15;71(2):430–438. doi: 10.1002/1097-0142(19930115)71:2<430::aid-cncr2820710225>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Whitehead R. H., Macrae F. A., St John D. J., Ma J. A colon cancer cell line (LIM1215) derived from a patient with inherited nonpolyposis colorectal cancer. J Natl Cancer Inst. 1985 Apr;74(4):759–765. [PubMed] [Google Scholar]
- Whitehead R. H., Zhang H. H., Hayward I. P. Retention of tissue-specific phenotype in a panel of colon carcinoma cell lines: relationship to clinical correlates. Immunol Cell Biol. 1992 Aug;70(Pt 4):227–236. doi: 10.1038/icb.1992.30. [DOI] [PubMed] [Google Scholar]
- Xing P. X., Prenzoska J., Layton G. T., Devine P. L., McKenzie I. F. Second-generation monoclonal antibodies to intestinal MUC2 peptide reactive with colon cancer. J Natl Cancer Inst. 1992 May 6;84(9):699–703. doi: 10.1093/jnci/84.9.699. [DOI] [PubMed] [Google Scholar]
- Xing P. X., Reynolds K., Tjandra J. J., Tang X. L., McKenzie I. F. Synthetic peptides reactive with anti-human milk fat globule membrane monoclonal antibodies. Cancer Res. 1990 Jan 1;50(1):89–96. [PubMed] [Google Scholar]
- Xing P. X., Tjandra J. J., Stacker S. A., Teh J. G., Thompson C. H., McLaughlin P. J., McKenzie I. F. Monoclonal antibodies reactive with mucin expressed in breast cancer. Immunol Cell Biol. 1989 Jun;67(Pt 3):183–195. doi: 10.1038/icb.1989.29. [DOI] [PubMed] [Google Scholar]
- Yan P. S., Ho S. B., Itzkowitz S. H., Byrd J. C., Siddiqui B., Kim Y. S. Expression of native and deglycosylated colon cancer mucin antigens in normal and malignant epithelial tissues. Lab Invest. 1990 Nov;63(5):698–706. [PubMed] [Google Scholar]
- Zegers N., Gerritse K., Deen C., Boersma W., Claassen E. An improved conjugation method for controlled covalent coupling of synthetic peptides to proteins using glutaraldehyde in a dialysis method. J Immunol Methods. 1990 Jul 3;130(2):195–200. doi: 10.1016/0022-1759(90)90048-z. [DOI] [PubMed] [Google Scholar]