Abstract
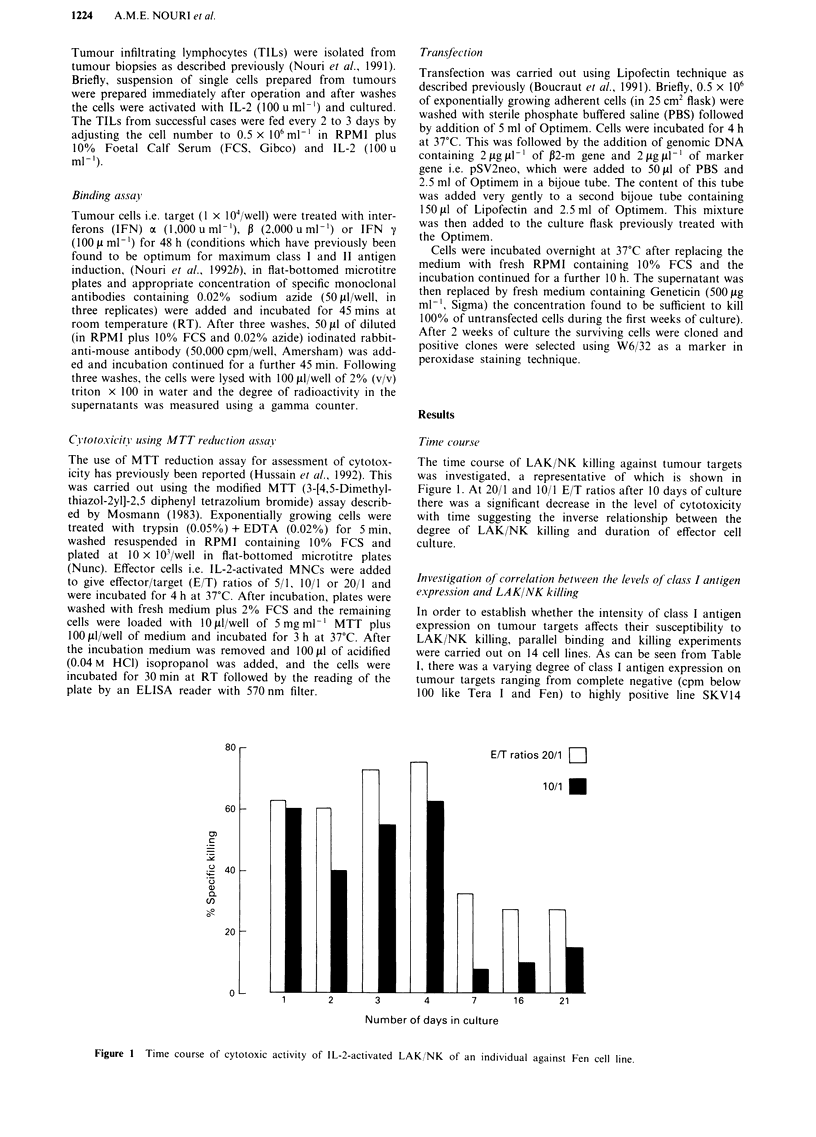
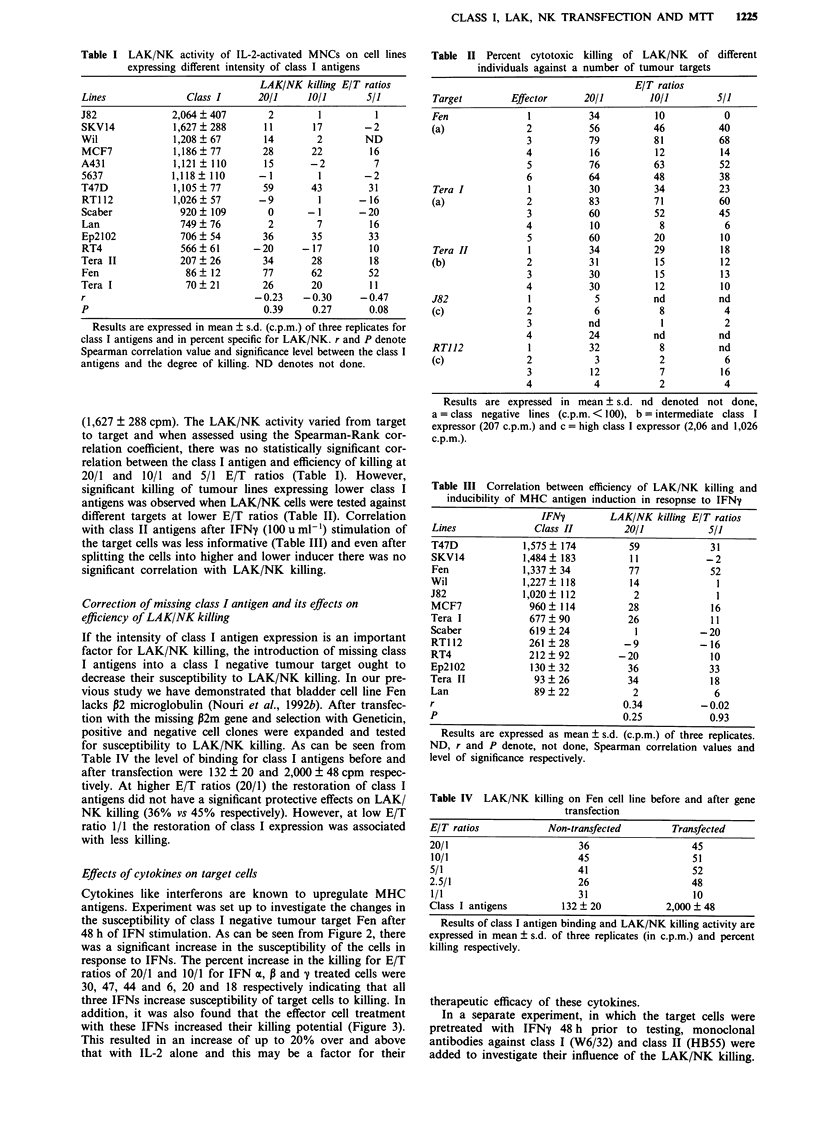
A modified tetrazolium reduction assay (MTT) was used to assess the relation between HLA class I antigen expression on tumour cells and their susceptibility as a target for non-MHC restricted LAK/NK cytotoxicity using interleukin-2 activated peripheral blood mononuclear cells (MNC) from normal individuals. At 20/1 effector/target ratio this ranged from no killing to 77%. The efficiency of killing was dependent on duration of effector cell culture with IL-2, peaking at day 10 and declining thereafter. This killing could be enhanced by addition of other cytokines including interferons alpha, beta and gamma. Study of a panel of 15 tumour cell lines using a single effector showed that there was no statistically significant inverse correlation (using Spearman rank test) between the degree of tumour class I expression and LAK/NK killing at 20/1 (r = 0.23 P = 0.39) and 10/1 (r = 0.30, P = 0.27) and at 5/1 E/T ratio r = 0.47, P = 0.08) respectively. Lack of inverse correlation between these two parameters came from study of one bladder tumour line (FEN), whose absent class I antigens had been corrected by transfection with beta 2 microglobulin gene. At high E/T ratio (20/1) there was an increase in the susceptibility of target cells to lysis (36% parent cell, 45% transfected cell), whilst at lower E/T ratios (1/1) there was significantly more killing of the non-transfected cells (10% vs 31%). The addition of anti-class I antibody W6/32 increased killing by 18% but this was non-specific as the same increase occurred with a class II antibody. These data suggest that overall there was not an inverse correlation between class I expression and LAK/NK killing at high E/T ratios, whilst at low (5/1 or lower) E/T ratios this correlation nearly reached statistical significance suggesting that the conflicting literature reports may be due to a threshold levels of effector cells above which the masking effects of MHC antigens disappears.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. A., Bennicelli J., Guerry D., 4th Defective antigen presentation by human melanoma cell lines cultured from advanced, but not biologically early, disease. J Immunol. 1989 Jun 1;142(11):4070–4078. [PubMed] [Google Scholar]
- Aosai F., Ohlen C., Ljunggren H. G., Höglund P., Franksson L., Ploegh H., Townsend A., Kärre K., Stauss H. J. Different types of allospecific CTL clones identified by their ability to recognize peptide loading-defective target cells. Eur J Immunol. 1991 Nov;21(11):2767–2774. doi: 10.1002/eji.1830211118. [DOI] [PubMed] [Google Scholar]
- Archimbaud E., Bailly M., Doré J. F. Inducibility of lymphokine activated killer (LAK) cells in patients with acute myelogenous leukaemia in complete remission and its clinical relevance. Br J Haematol. 1991 Mar;77(3):328–334. doi: 10.1111/j.1365-2141.1991.tb08579.x. [DOI] [PubMed] [Google Scholar]
- Belldegrun A., Muul L. M., Rosenberg S. A. Interleukin 2 expanded tumor-infiltrating lymphocytes in human renal cell cancer: isolation, characterization, and antitumor activity. Cancer Res. 1988 Jan 1;48(1):206–214. [PubMed] [Google Scholar]
- Boucraut J., Hakem R., Gauthier A., Fauchet R., Le Bouteiller P. Transfected trophoblast-derived human cells can express a single HLA class I allelic product. Tissue Antigens. 1991 Feb;37(2):84–89. doi: 10.1111/j.1399-0039.1991.tb01850.x. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P., Barnstable C. J., Crumpton M. J., Bodmer W. F. Monoclonal antibodies for analysis of the HLA system. Immunol Rev. 1979;47:3–61. doi: 10.1111/j.1600-065x.1979.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Carlson G. A., Wegmann T. G. Rapid in vivo destruction of semi-syngeneic and allogeneic cells by nonimmunized mice as a consequence of nonidentity at H-2. J Immunol. 1977 Jun;118(6):2130–2137. [PubMed] [Google Scholar]
- Gansbacher B., Zier K., Daniels B., Cronin K., Bannerji R., Gilboa E. Interleukin 2 gene transfer into tumor cells abrogates tumorigenicity and induces protective immunity. J Exp Med. 1990 Oct 1;172(4):1217–1224. doi: 10.1084/jem.172.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik E., Gunji Y., Herberman R. B. H-2 antigen expression and sensitivity of BL6 melanoma cells to natural killer cell cytotoxicity. J Immunol. 1988 Mar 15;140(6):2096–2102. [PubMed] [Google Scholar]
- Grimm E. A., Mazumder A., Zhang H. Z., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982 Jun 1;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui K., Grosveld F., Festenstein H. Rejection of transplantable AKR leukaemia cells following MHC DNA-mediated cell transformation. Nature. 1984 Oct 25;311(5988):750–752. doi: 10.1038/311750a0. [DOI] [PubMed] [Google Scholar]
- Hussain R. F., Nouri A. M., Oliver R. T. A new approach for measurement of cytotoxicity using colorimetric assay. J Immunol Methods. 1993 Mar 15;160(1):89–96. doi: 10.1016/0022-1759(93)90012-v. [DOI] [PubMed] [Google Scholar]
- Itoh K., Platsoucas C. D., Balch C. M. Autologous tumor-specific cytotoxic T lymphocytes in the infiltrate of human metastatic melanomas. Activation by interleukin 2 and autologous tumor cells, and involvement of the T cell receptor. J Exp Med. 1988 Oct 1;168(4):1419–1441. doi: 10.1084/jem.168.4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975 Feb;5(2):112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- Kärre K., Ljunggren H. G., Piontek G., Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986 Feb 20;319(6055):675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- Lampson L. A., Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980 Jul;125(1):293–299. [PubMed] [Google Scholar]
- Lee S. K., Oliver R. T. Autologous leukemia-specific T-cell-mediated lymphocytotoxicity in patients with acute myelogenous leukemia. J Exp Med. 1978 Mar 1;147(3):912–922. doi: 10.1084/jem.147.3.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo P. I., Spencer C. E. Use of anti-HLA antibodies to mask major histocompatibility complex gene products on tumor cells can enhance susceptibility of these cells to lysis by natural killer cells. J Clin Invest. 1989 Jan;83(1):278–287. doi: 10.1172/JCI113870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maziarz R. T., Mentzer S. J., Burakoff S. J., Faller D. V. Distinct effects of interferon-gamma and MHC class I surface antigen levels on resistance of the K562 tumor cell line to natural killer-mediated lysis. Cell Immunol. 1990 Oct 15;130(2):329–338. doi: 10.1016/0008-8749(90)90276-w. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Gotch F. M., Santos-Aguado J., Strominger J. L. Effect of mutations and variations of HLA-A2 on recognition of a virus peptide epitope by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9194–9198. doi: 10.1073/pnas.85.23.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Moss P. A., Moots R. J., Rosenberg W. M., Rowland-Jones S. J., Bodmer H. C., McMichael A. J., Bell J. I. Extensive conservation of alpha and beta chains of the human T-cell antigen receptor recognizing HLA-A2 and influenza A matrix peptide. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8987–8990. doi: 10.1073/pnas.88.20.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulé J. J., Shu S., Schwarz S. L., Rosenberg S. A. Adoptive immunotherapy of established pulmonary metastases with LAK cells and recombinant interleukin-2. Science. 1984 Sep 28;225(4669):1487–1489. doi: 10.1126/science.6332379. [DOI] [PubMed] [Google Scholar]
- Nouri A. M., Bergbaum A., Lederer E., Crosby D., Shamsa A., Oliver R. T. Paired tumour infiltrating lymphocyte (TIL) and tumour cell line from bladder cancer: a new approach to study tumour immunology in vitro. Eur J Cancer. 1991;27(5):608–612. doi: 10.1016/0277-5379(91)90241-5. [DOI] [PubMed] [Google Scholar]
- Oliver R. T., Nouri A. M. T cell immune response to cancer in humans and its relevance for immunodiagnosis and therapy. Cancer Surv. 1992;13:173–204. [PubMed] [Google Scholar]
- Pena J., Solana R., Alonso M. C., Santamaria M., Serrano R., Ramirez R., Carracedo J. MHC class I expression on human tumour cells and their susceptibility to NK lysis. J Immunogenet. 1989 Aug-Oct;16(4-5):407–412. doi: 10.1111/j.1744-313x.1989.tb00488.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Aebersold P., Cornetta K., Kasid A., Morgan R. A., Moen R., Karson E. M., Lotze M. T., Yang J. C., Topalian S. L. Gene transfer into humans--immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990 Aug 30;323(9):570–578. doi: 10.1056/NEJM199008303230904. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Yang J. C., Linehan W. M., Seipp C., Calabro S., Karp S. E., Sherry R. M., Steinberg S., White D. E. Combination therapy with interleukin-2 and alpha-interferon for the treatment of patients with advanced cancer. J Clin Oncol. 1989 Dec;7(12):1863–1874. doi: 10.1200/JCO.1989.7.12.1863. [DOI] [PubMed] [Google Scholar]
- Storkus W. J., Salter R. D., Alexander J., Ward F. E., Ruiz R. E., Cresswell P., Dawson J. R. Class I-induced resistance to natural killing: identification of nonpermissive residues in HLA-A2. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):5989–5992. doi: 10.1073/pnas.88.14.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]


