Abstract
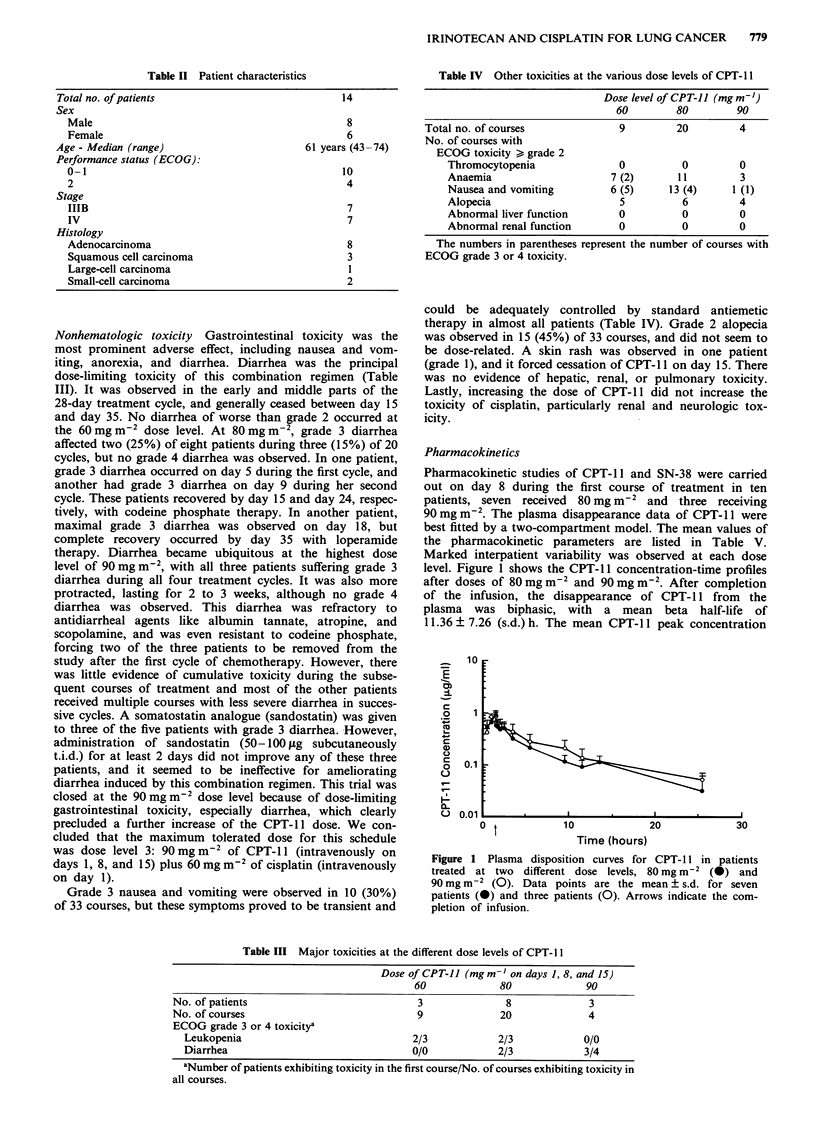
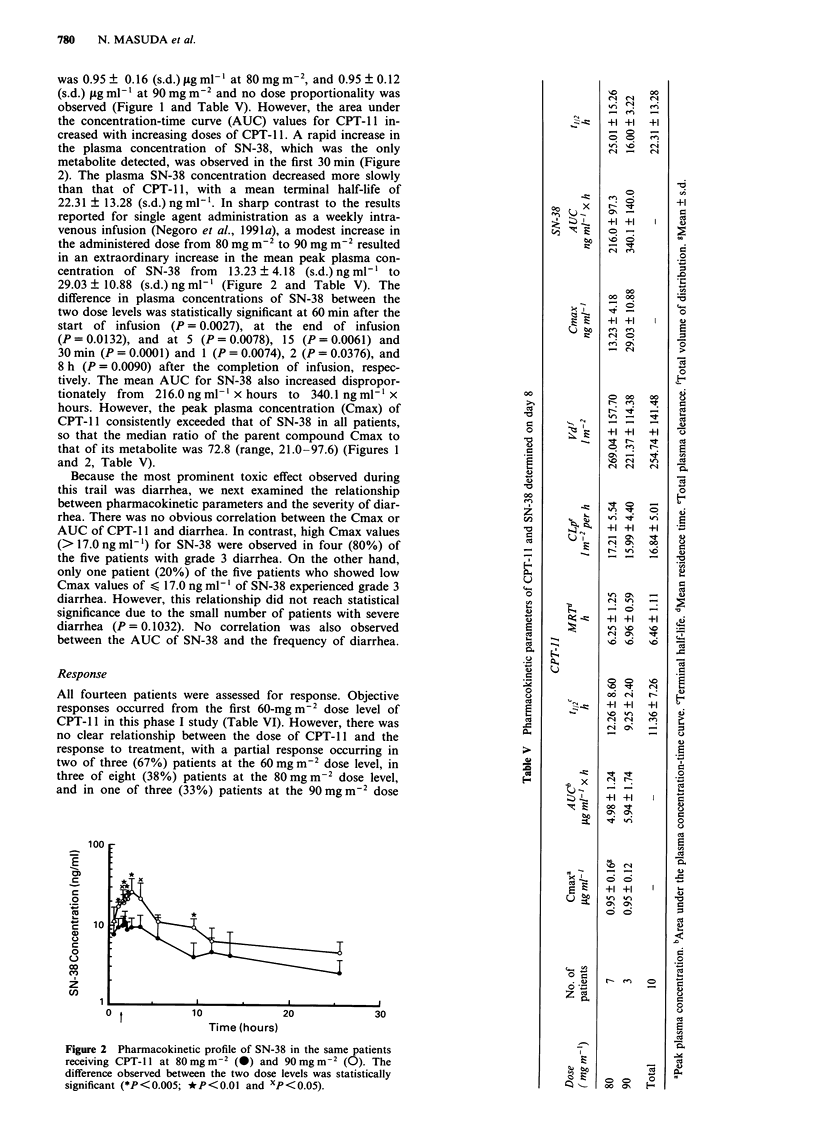
We have conducted a Phase I trial to determine the maximum tolerated dose of CPT-11 together with a fixed dose of cisplatin in patients with advanced lung cancer, and the dose-limiting toxicities of this combination. Fourteen previously untreated patients with stage IIIB or IV disease were treated with CPT-11 (90-min intravenous infusion on days 1, 8, and 15) plus cisplatin (60 mg m-2, intravenously on day 1). The starting dose of CPT-11 was 60 mg m-2, and diarrhea was the dose-limiting toxicity at the 90 mg m-2 dose level. All three patients (all four cycles) given 90 mg m-2 of CPT-11 experienced grade 3 diarrhea. Hematologic toxicity was relatively mild. Elimination of CPT-11 was biphasic with a mean (+/- s.d.) beta half-life of 11.36 +/- 7.26 h. The mean terminal half-life of the major metabolite (7-ethyl-10-hydroxycamptothecin; SN-38) was 22.13 +/- 13.28 (s.d.) h, and modest escalation of the CPT-11 dose from 80 mg m-2 to 90 mg m-2 resulted in a statistically significant apparent increase in the plasma concentrations of SN-38. There were one complete response (7%) and five partial responses (36%) among the 14 patients for an overall response rate of 43%. The recommended dose for Phase II studies is 80 mg m-2 of CPT-11 and 60 mg m-2 of cisplatin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fukuoka M., Niitani H., Suzuki A., Motomiya M., Hasegawa K., Nishiwaki Y., Kuriyama T., Ariyoshi Y., Negoro S., Masuda N. A phase II study of CPT-11, a new derivative of camptothecin, for previously untreated non-small-cell lung cancer. J Clin Oncol. 1992 Jan;10(1):16–20. doi: 10.1200/JCO.1992.10.1.16. [DOI] [PubMed] [Google Scholar]
- Hertzberg R. P., Caranfa M. J., Hecht S. M. On the mechanism of topoisomerase I inhibition by camptothecin: evidence for binding to an enzyme-DNA complex. Biochemistry. 1989 May 30;28(11):4629–4638. doi: 10.1021/bi00437a018. [DOI] [PubMed] [Google Scholar]
- Hsiang Y. H., Hertzberg R., Hecht S., Liu L. F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985 Nov 25;260(27):14873–14878. [PubMed] [Google Scholar]
- Hsiang Y. H., Liu L. F. Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res. 1988 Apr 1;48(7):1722–1726. [PubMed] [Google Scholar]
- Kaneda N., Nagata H., Furuta T., Yokokura T. Metabolism and pharmacokinetics of the camptothecin analogue CPT-11 in the mouse. Cancer Res. 1990 Mar 15;50(6):1715–1720. [PubMed] [Google Scholar]
- Kunimoto T., Nitta K., Tanaka T., Uehara N., Baba H., Takeuchi M., Yokokura T., Sawada S., Miyasaka T., Mutai M. Antitumor activity of 7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxy-camptothec in, a novel water-soluble derivative of camptothecin, against murine tumors. Cancer Res. 1987 Nov 15;47(22):5944–5947. [PubMed] [Google Scholar]
- Masuda N., Fukuoka M., Kusunoki Y., Matsui K., Takifuji N., Kudoh S., Negoro S., Nishioka M., Nakagawa K., Takada M. CPT-11: a new derivative of camptothecin for the treatment of refractory or relapsed small-cell lung cancer. J Clin Oncol. 1992 Aug;10(8):1225–1229. doi: 10.1200/JCO.1992.10.8.1225. [DOI] [PubMed] [Google Scholar]
- Masuda N., Fukuoka M., Takada M., Kusunoki Y., Negoro S., Matsui K., Kudoh S., Takifuji N., Nakagawa K., Kishimoto S. CPT-11 in combination with cisplatin for advanced non-small-cell lung cancer. J Clin Oncol. 1992 Nov;10(11):1775–1780. doi: 10.1200/JCO.1992.10.11.1775. [DOI] [PubMed] [Google Scholar]
- Matsuzaki T., Yokokura T., Mutai M., Tsuruo T. Inhibition of spontaneous and experimental metastasis by a new derivative of camptothecin, CPT-11, in mice. Cancer Chemother Pharmacol. 1988;21(4):308–312. doi: 10.1007/BF00264196. [DOI] [PubMed] [Google Scholar]
- Mountain C. F. A new international staging system for lung cancer. Chest. 1986 Apr;89(4 Suppl):225S–233S. doi: 10.1378/chest.89.4_supplement.225s. [DOI] [PubMed] [Google Scholar]
- Negoro S., Fukuoka M., Masuda N., Takada M., Kusunoki Y., Matsui K., Takifuji N., Kudoh S., Niitani H., Taguchi T. Phase I study of weekly intravenous infusions of CPT-11, a new derivative of camptothecin, in the treatment of advanced non-small-cell lung cancer. J Natl Cancer Inst. 1991 Aug 21;83(16):1164–1168. doi: 10.1093/jnci/83.16.1164. [DOI] [PubMed] [Google Scholar]
- Ohno R., Okada K., Masaoka T., Kuramoto A., Arima T., Yoshida Y., Ariyoshi H., Ichimaru M., Sakai Y., Oguro M. An early phase II study of CPT-11: a new derivative of camptothecin, for the treatment of leukemia and lymphoma. J Clin Oncol. 1990 Nov;8(11):1907–1912. doi: 10.1200/JCO.1990.8.11.1907. [DOI] [PubMed] [Google Scholar]
- Tsuji T., Kaneda N., Kado K., Yokokura T., Yoshimoto T., Tsuru D. CPT-11 converting enzyme from rat serum: purification and some properties. J Pharmacobiodyn. 1991 Jun;14(6):341–349. doi: 10.1248/bpb1978.14.341. [DOI] [PubMed] [Google Scholar]


