Abstract
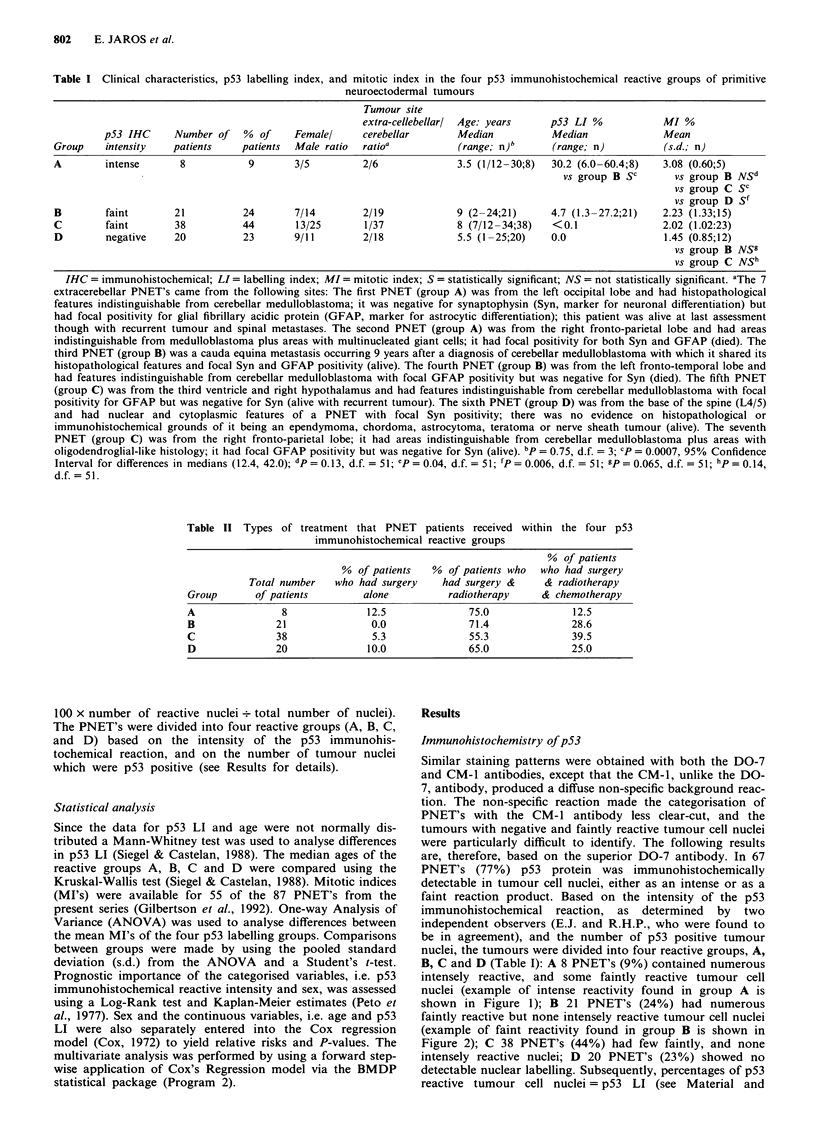
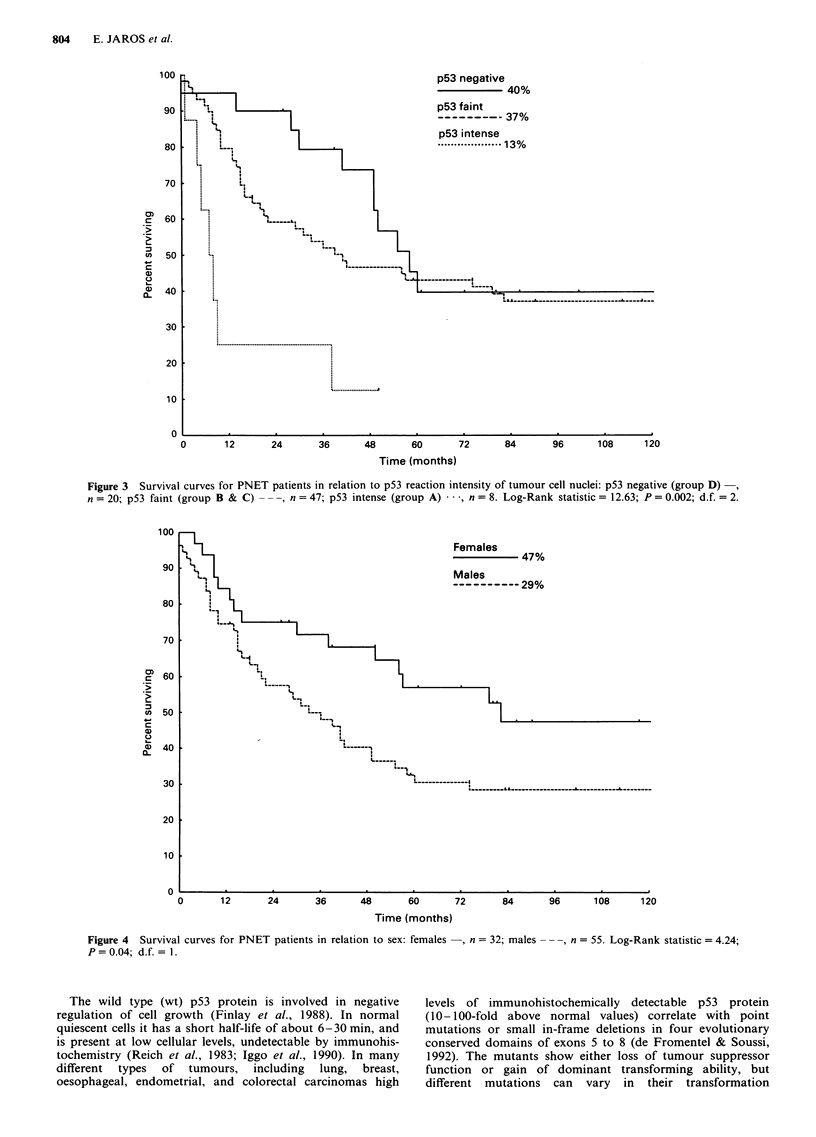
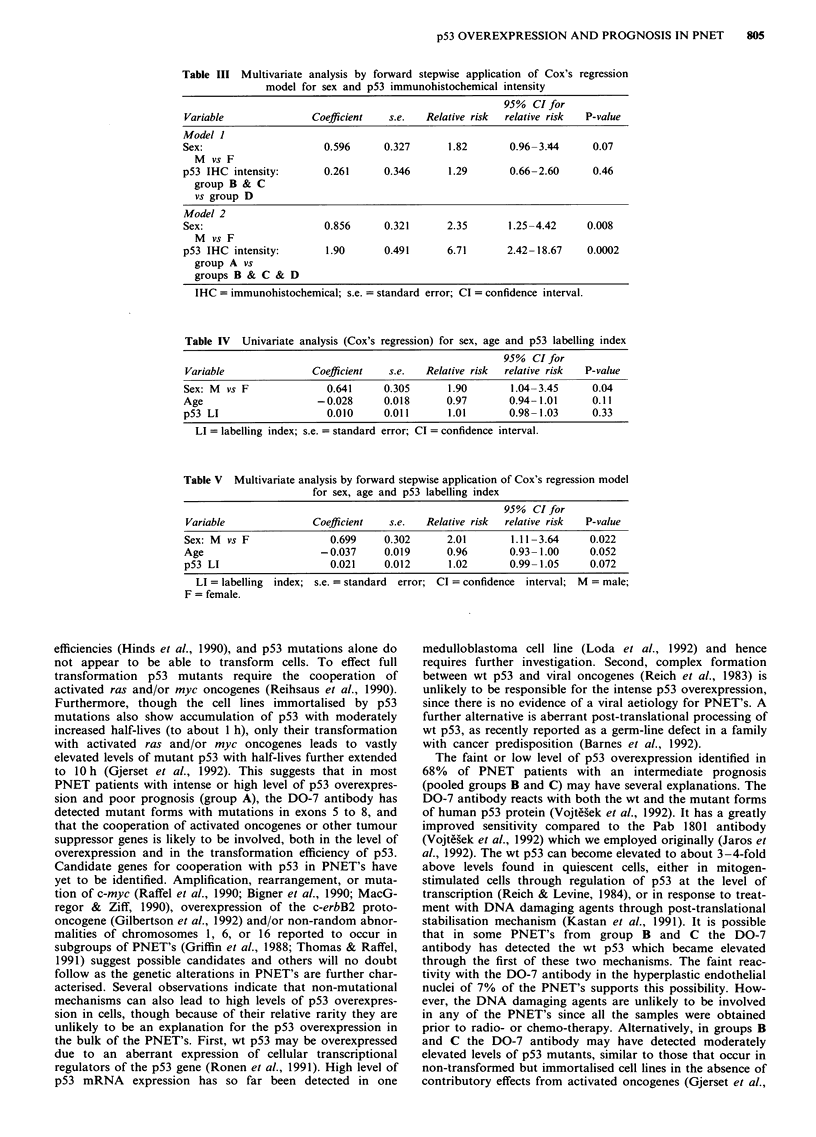
Primitive neuroectodermal tumours (PNET's) or medulloblastomas are common primary brain tumours of childhood. Current treatment protocols achieve 50-60% cures. However, it has proved difficult to develop better treatment for the remaining patients because prognostic factors are not established. We have investigated the prognostic value of p53 protein expression in 87 PNET's using immunohistochemistry with DO-7 and CM-1 antibodies on biopsy paraffin sections. Eight patients (9%) had intensely reactive tumour cell nuclei, and a significantly reduced survival (P = 0.002); only one survives and this with a recurrent tumour 50 months following diagnosis. Sixty eight per cent of patients had faintly reactive tumour cell nuclei, a reduced survival up to 4 years but a long term survival not significantly different (P = 0.41) from 23% of patients with p53 negative PNET's; the 10 year survival rates were 37% and 40%, respectively. Males had a reduced survival (P = 0.04) with a 2-fold relative risk of death compared to females. Multivariate analysis showed that intense overexpression of p53 protein identifies a group of PNET patients with a 7-fold relative risk of death compared to all other cases, irrespective of sex. This marked difference suggests the involvement of p53 in the pathogenesis of PNET's which have a particularly poor response to treatment, and should help to develop new therapies for this group of patients.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbareschi M., Iuzzolino P., Pennella A., Allegranza A., Arrigoni G., Dalla Palma P., Doglioni C. p53 protein expression in central nervous system neoplasms. J Clin Pathol. 1992 Jul;45(7):583–586. doi: 10.1136/jcp.45.7.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D. M., Hanby A. M., Gillett C. E., Mohammed S., Hodgson S., Bobrow L. G., Leigh I. M., Purkis T., MacGeoch C., Spurr N. K. Abnormal expression of wild type p53 protein in normal cells of a cancer family patient. Lancet. 1992 Aug 1;340(8814):259–263. doi: 10.1016/0140-6736(92)92354-i. [DOI] [PubMed] [Google Scholar]
- Bigner S. H., Friedman H. S., Vogelstein B., Oakes W. J., Bigner D. D. Amplification of the c-myc gene in human medulloblastoma cell lines and xenografts. Cancer Res. 1990 Apr 15;50(8):2347–2350. [PubMed] [Google Scholar]
- Bodner S. M., Minna J. D., Jensen S. M., D'Amico D., Carbone D., Mitsudomi T., Fedorko J., Buchhagen D. L., Nau M. M., Gazdar A. F. Expression of mutant p53 proteins in lung cancer correlates with the class of p53 gene mutation. Oncogene. 1992 Apr;7(4):743–749. [PubMed] [Google Scholar]
- Caputy A. J., McCullough D. C., Manz H. J., Patterson K., Hammock M. K. A review of the factors influencing the prognosis of medulloblastoma. The importance of cell differentiation. J Neurosurg. 1987 Jan;66(1):80–87. doi: 10.3171/jns.1987.66.1.0080. [DOI] [PubMed] [Google Scholar]
- Caron de Fromentel C., Soussi T. TP53 tumor suppressor gene: a model for investigating human mutagenesis. Genes Chromosomes Cancer. 1992 Jan;4(1):1–15. doi: 10.1002/gcc.2870040102. [DOI] [PubMed] [Google Scholar]
- Clarke A. R., Purdie C. A., Harrison D. J., Morris R. G., Bird C. C., Hooper M. L., Wyllie A. H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993 Apr 29;362(6423):849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Tan T. H., Eliyahu D., Oren M., Levine A. J. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol. 1988 Feb;8(2):531–539. doi: 10.1128/mcb.8.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson R. J., Jaros E. B., Perry R. H., Pearson A. D. Prognostic factors in medulloblastoma. Lancet. 1992 Aug 22;340(8817):480–480. doi: 10.1016/0140-6736(92)91797-c. [DOI] [PubMed] [Google Scholar]
- Gjerset R. A., Arya J., Volkman S., Haas M. Association of induction of a fully tumorigenic phenotype in murine radiation-induced T-lymphoma cells with loss of differentiation antigens, gain of CD44, and alterations in p53 protein levels. Mol Carcinog. 1992;5(3):190–198. doi: 10.1002/mc.2940050305. [DOI] [PubMed] [Google Scholar]
- Griffin C. A., Hawkins A. L., Packer R. J., Rorke L. B., Emanuel B. S. Chromosome abnormalities in pediatric brain tumors. Cancer Res. 1988 Jan 1;48(1):175–180. [PubMed] [Google Scholar]
- Hinds P. W., Finlay C. A., Quartin R. S., Baker S. J., Fearon E. R., Vogelstein B., Levine A. J. Mutant p53 DNA clones from human colon carcinomas cooperate with ras in transforming primary rat cells: a comparison of the "hot spot" mutant phenotypes. Cell Growth Differ. 1990 Dec;1(12):571–580. [PubMed] [Google Scholar]
- Iggo R., Gatter K., Bartek J., Lane D., Harris A. L. Increased expression of mutant forms of p53 oncogene in primary lung cancer. Lancet. 1990 Mar 24;335(8691):675–679. doi: 10.1016/0140-6736(90)90801-b. [DOI] [PubMed] [Google Scholar]
- Ito S., Hoshino T., Prados M. D., Edwards M. S. Cell kinetics of medulloblastomas. Cancer. 1992 Aug 1;70(3):671–678. doi: 10.1002/1097-0142(19920801)70:3<671::aid-cncr2820700322>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- James C. D., He J., Carlbom E., Mikkelsen T., Ridderheim P. A., Cavenee W. K., Collins V. P. Loss of genetic information in central nervous system tumors common to children and young adults. Genes Chromosomes Cancer. 1990 Jul;2(2):94–102. doi: 10.1002/gcc.2870020204. [DOI] [PubMed] [Google Scholar]
- Jaros E., Perry R. H., Adam L., Kelly P. J., Crawford P. J., Kalbag R. M., Mendelow A. D., Sengupta R. P., Pearson A. D. Prognostic implications of p53 protein, epidermal growth factor receptor, and Ki-67 labelling in brain tumours. Br J Cancer. 1992 Aug;66(2):373–385. doi: 10.1038/bjc.1992.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Lane D. P. Cancer. A death in the life of p53. Nature. 1993 Apr 29;362(6423):786–787. doi: 10.1038/362786a0. [DOI] [PubMed] [Google Scholar]
- Lane D. P. Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Loda M., Giangaspero F., Badiali M., Capodieci P., Pession A. p53 gene expression in medulloblastoma by quantitative polymerase chain reaction. Diagn Mol Pathol. 1992 Mar;1(1):36–44. doi: 10.1097/00019606-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- MacGregor D. N., Ziff E. B. Elevated c-myc expression in childhood medulloblastomas. Pediatr Res. 1990 Jul;28(1):63–68. doi: 10.1203/00006450-199007000-00014. [DOI] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffel C., Gilles F. E., Weinberg K. I. Reduction to homozygosity and gene amplification in central nervous system primitive neuroectodermal tumors of childhood. Cancer Res. 1990 Feb 1;50(3):587–591. [PubMed] [Google Scholar]
- Reich N. C., Levine A. J. Growth regulation of a cellular tumour antigen, p53, in nontransformed cells. Nature. 1984 Mar 8;308(5955):199–201. doi: 10.1038/308199a0. [DOI] [PubMed] [Google Scholar]
- Reich N. C., Oren M., Levine A. J. Two distinct mechanisms regulate the levels of a cellular tumor antigen, p53. Mol Cell Biol. 1983 Dec;3(12):2143–2150. doi: 10.1128/mcb.3.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reihsaus E., Kohler M., Kraiss S., Oren M., Montenarh M. Regulation of the level of the oncoprotein p53 in non-transformed and transformed cells. Oncogene. 1990 Jan;5(1):137–145. [PubMed] [Google Scholar]
- Ronen D., Rotter V., Reisman D. Expression from the murine p53 promoter is mediated by factor binding to a downstream helix-loop-helix recognition motif. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4128–4132. doi: 10.1073/pnas.88.10.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorke L. B., Gilles F. H., Davis R. L., Becker L. E. Revision of the World Health Organization classification of brain tumors for childhood brain tumors. Cancer. 1985 Oct 1;56(7 Suppl):1869–1886. doi: 10.1002/1097-0142(19851001)56:7+<1869::aid-cncr2820561330>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Solomon E., Barker D. F. Report of the committee on the genetic constitution of chromosome 17. Cytogenet Cell Genet. 1989;51(1-4):319–337. doi: 10.1159/000132797. [DOI] [PubMed] [Google Scholar]
- Tait D. M., Thornton-Jones H., Bloom H. J., Lemerle J., Morris-Jones P. Adjuvant chemotherapy for medulloblastoma: the first multi-centre control trial of the International Society of Paediatric Oncology (SIOP I). Eur J Cancer. 1990 Apr;26(4):464–469. [PubMed] [Google Scholar]
- Thomas G. A., Raffel C. Loss of heterozygosity on 6q, 16q, and 17p in human central nervous system primitive neuroectodermal tumors. Cancer Res. 1991 Jan 15;51(2):639–643. [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. p53 function and dysfunction. Cell. 1992 Aug 21;70(4):523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- Vojtesek B., Bártek J., Midgley C. A., Lane D. P. An immunochemical analysis of the human nuclear phosphoprotein p53. New monoclonal antibodies and epitope mapping using recombinant p53. J Immunol Methods. 1992 Jul 6;151(1-2):237–244. doi: 10.1016/0022-1759(92)90122-a. [DOI] [PubMed] [Google Scholar]




