Abstract
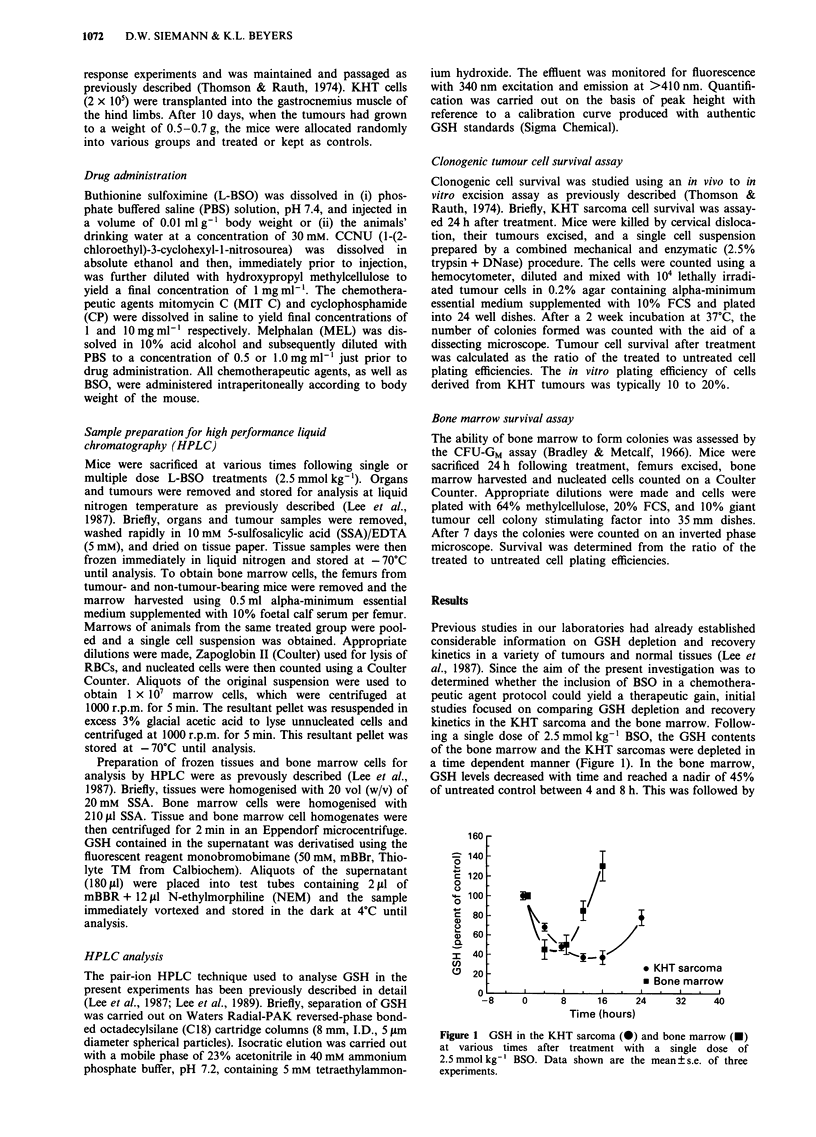
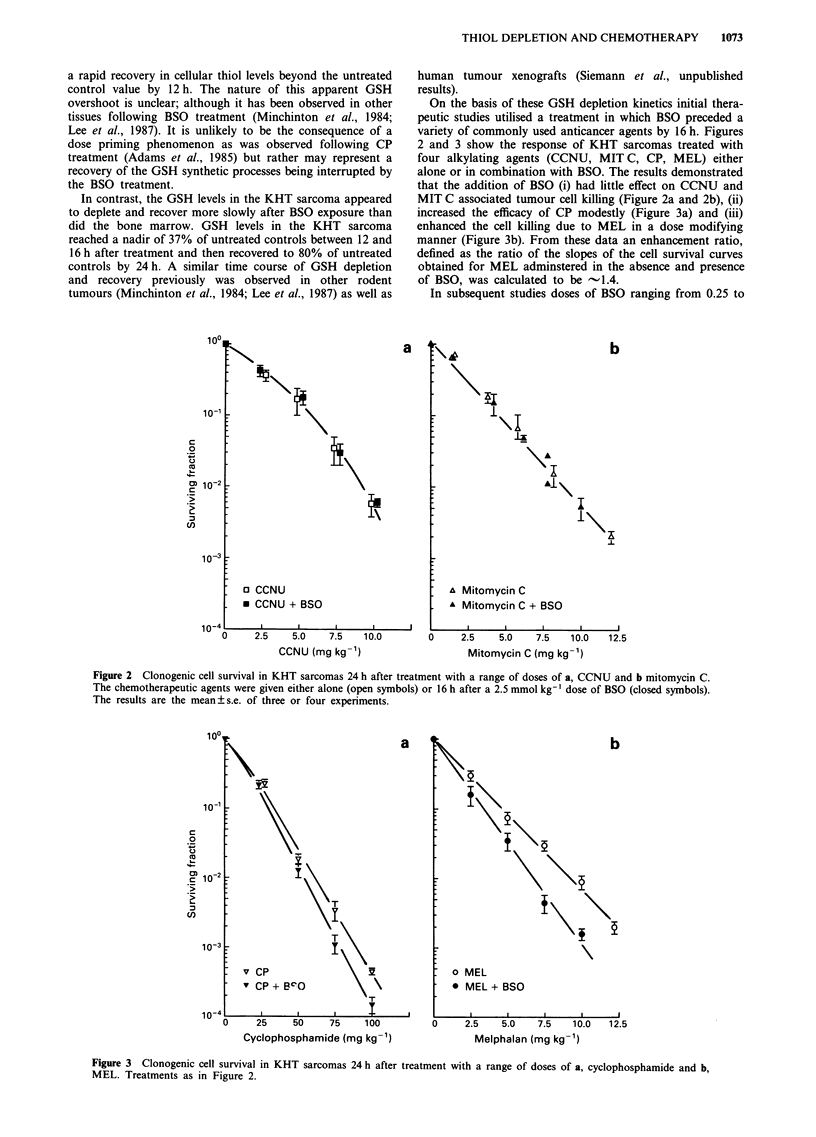
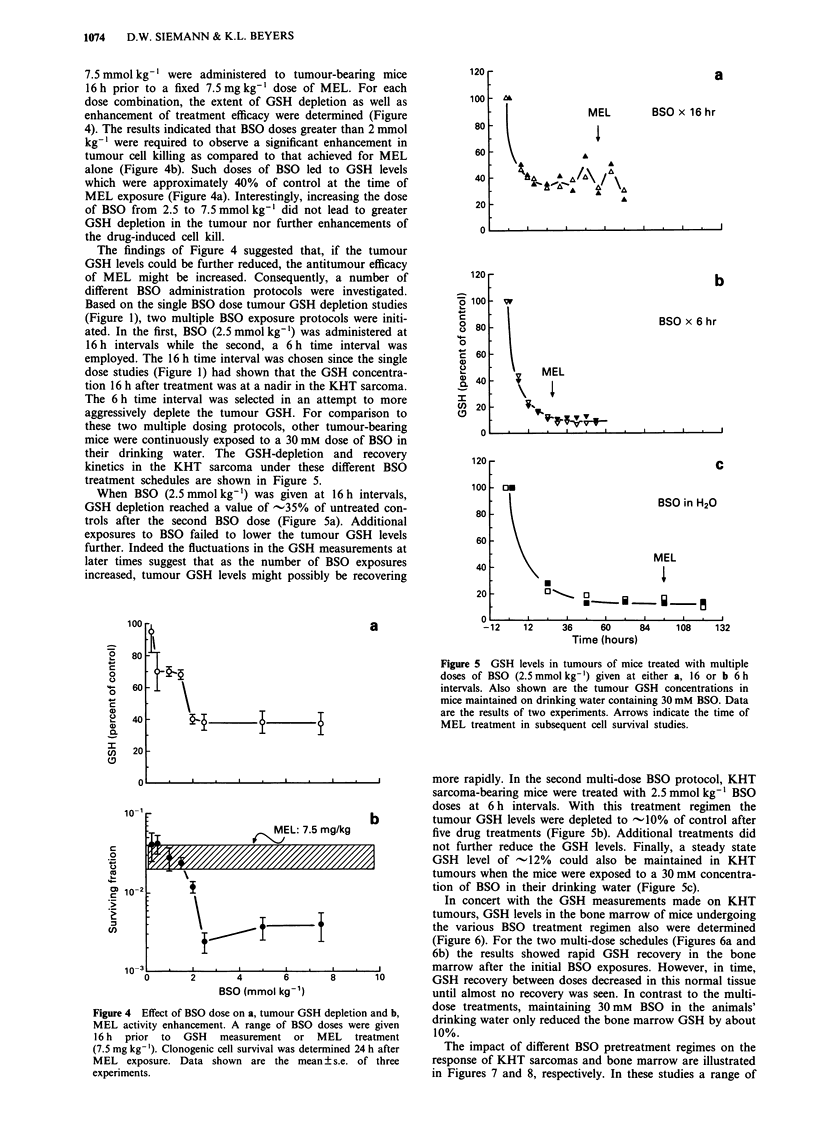
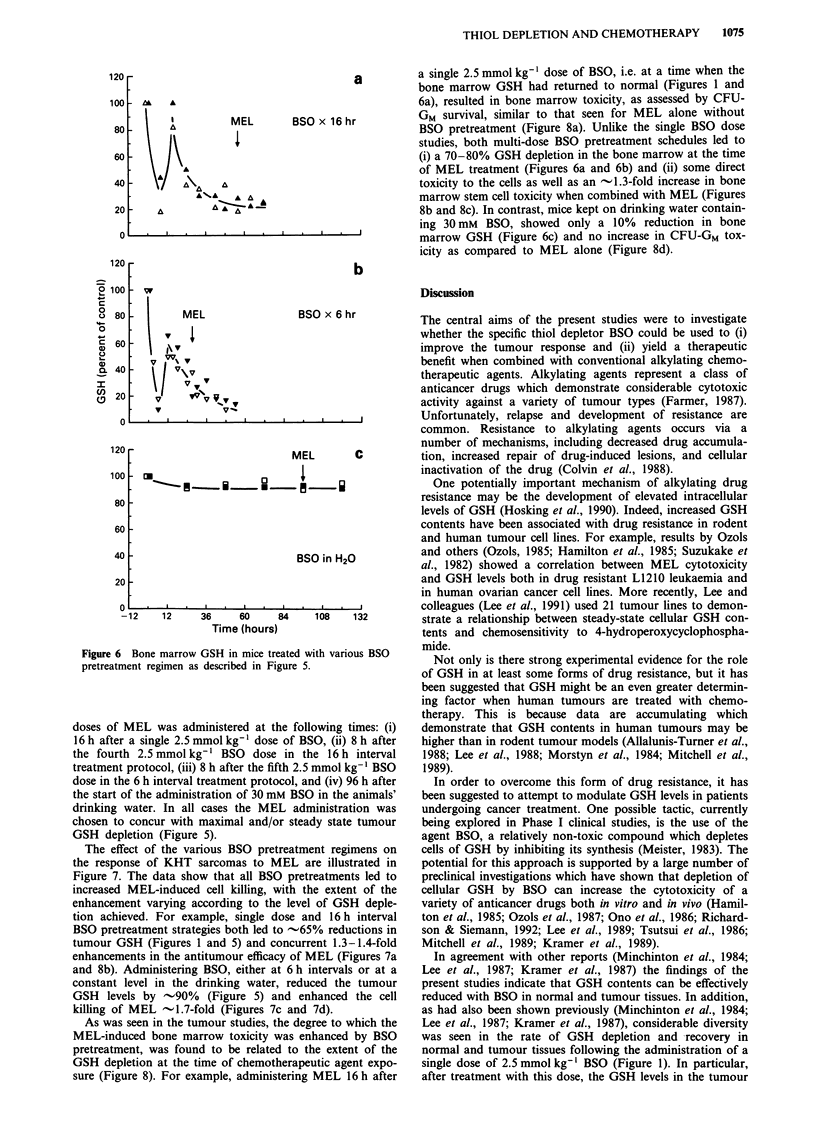
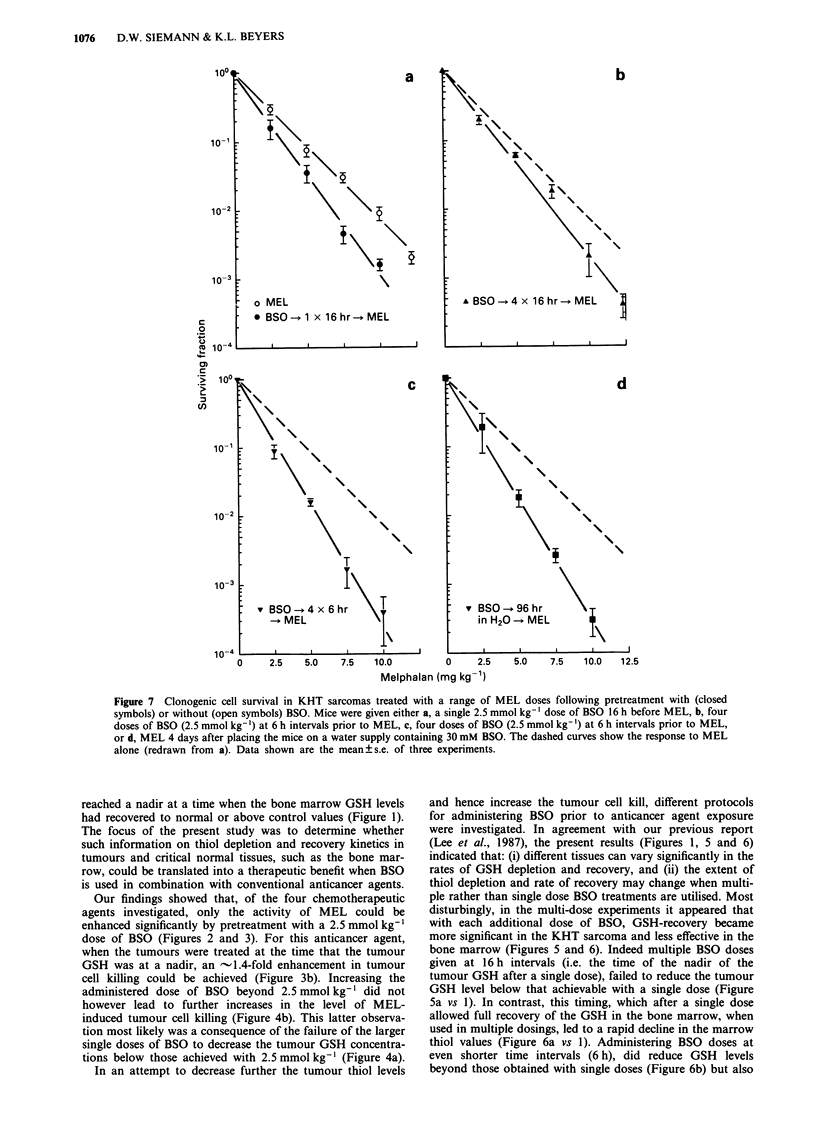
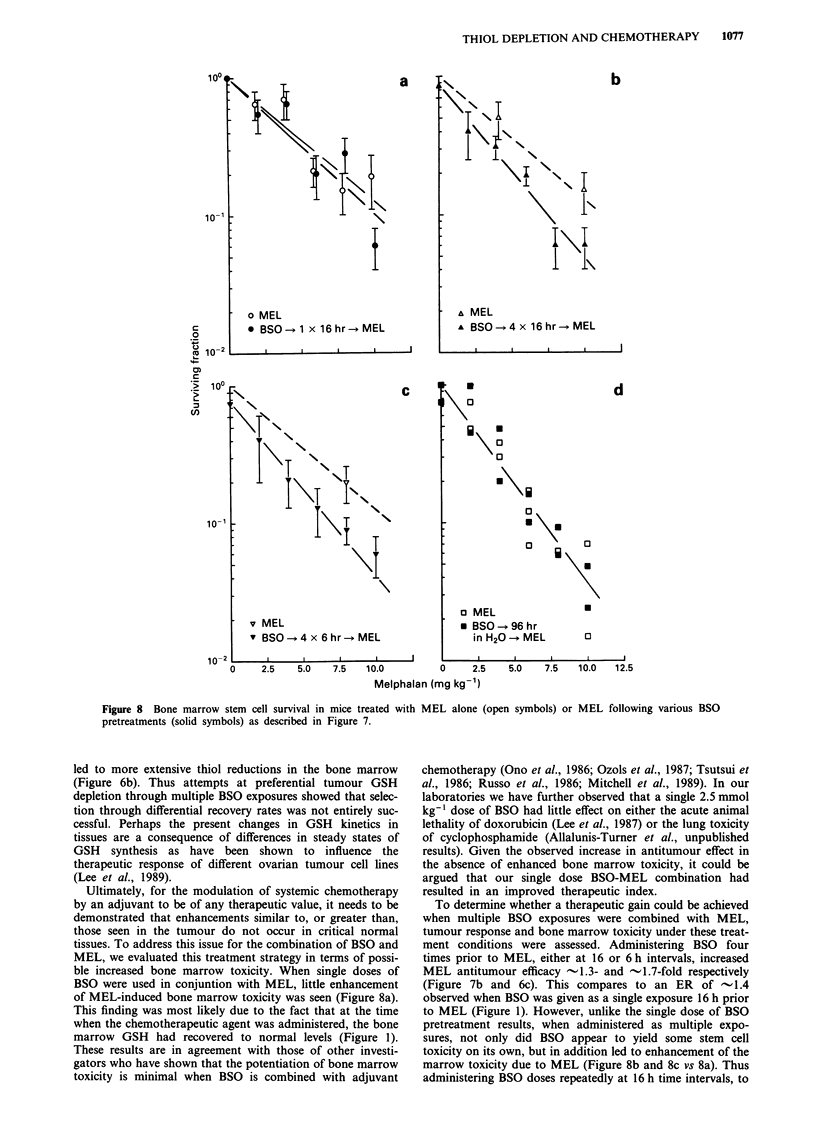
The effect of administering the thiol modulating agent buthionine sulfoximine (BSO) in conjunction with alkylating chemotherapy was investigated in vivo in the mouse KHT sarcomas and bone marrow stem cells. Tumour response to treatment was assessed by an in vivo to in vitro excision assay and bone marrow survival was determined in vitro by CFU-GM. Glutathione (GSH) depletion and recovery kinetics were determined at various times after treatment using high performance liquid chromatography (HPLC) techniques. Following a single 2.5 mmol kg-1 dose of BSO, tumour GSH reached a nadir of approximately 40% of control 12-16 h after treatment. Bone marrow GSH was depleted to approximately 45% of control 4-8 h after treatment but recovered to normal by 16 h. When a range of doses of CCNU, mitomycin C, cyclophosphamide or melphalan (MEL) were given 16 h after mice were exposed to a 2.5 mmol kg-1 dose of BSO, only the antitumour efficacy of MEL was effectively enhanced (by a factor of approximately 1.4). This BSO-MEL combination appeared to be selective for the tumour as the bone marrow toxicity was not increased beyond that seen for MEL alone. Since increasing the administered dose of BSO neither increased the extent of thiol depletion in the tumour nor enhanced the antitumour efficacy of MEL, three other protocols for delivering the thiol depletor were explored. BSO was given either as multiple 2.5 mmol kg-1 doses administered at 6 or 16 h intervals or continuously at a concentration of 30 mM supplied in the animals' drinking water. Both multi-dose BSO pretreatments were found to increase both the antitumour efficacy and normal tissue toxicity of MEL such that no advantage compared to the single dose combination was achieved. In contrast, maintaining the thiol depletor in the drinking water led to an approximately 1.7-fold increase in the antitumour efficacy of MEL without any corresponding increase in bone marrow stem cell toxicity. For the various pretreatment strategies it was possible, in all cases, to account for the presence or absence of a net therapeutic benefit on the basis of the tumour and bone marrow GSH depletion and recovery kinetics.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams K. J., Carmichael J., Wolf C. R. Altered mouse bone marrow glutathione and glutathione transferase levels in response to cytotoxins. Cancer Res. 1985 Apr;45(4):1669–1673. [PubMed] [Google Scholar]
- Allalunis-Turner M. J., Lee F. Y., Siemann D. W. Comparison of glutathione levels in rodent and human tumor cells grown in vitro and in vivo. Cancer Res. 1988 Jul 1;48(13):3657–3660. [PubMed] [Google Scholar]
- Arrick B. A., Nathan C. F. Glutathione metabolism as a determinant of therapeutic efficacy: a review. Cancer Res. 1984 Oct;44(10):4224–4232. [PubMed] [Google Scholar]
- Biaglow J. E., Varnes M. E., Clark E. P., Epp E. R. The role of thiols in cellular response to radiation and drugs. Radiat Res. 1983 Sep;95(3):437–455. [PubMed] [Google Scholar]
- Bradley T. R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966 Jun;44(3):287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- Colvin M., Russo J. E., Hilton J., Dulik D. M., Fenselau C. Enzymatic mechanisms of resistance to alkylating agents in tumor cells and normal tissues. Adv Enzyme Regul. 1988;27:211–221. doi: 10.1016/0065-2571(88)90018-0. [DOI] [PubMed] [Google Scholar]
- Crook T. R., Souhami R. L., Whyman G. D., McLean A. E. Glutathione depletion as a determinant of sensitivity of human leukemia cells to cyclophosphamide. Cancer Res. 1986 Oct;46(10):5035–5038. [PubMed] [Google Scholar]
- Farmer P. B. Metabolism and reactions of alkylating agents. Pharmacol Ther. 1987;35(3):301–358. doi: 10.1016/0163-7258(87)90099-4. [DOI] [PubMed] [Google Scholar]
- Hamilton T. C., Winker M. A., Louie K. G., Batist G., Behrens B. C., Tsuruo T., Grotzinger K. R., McKoy W. M., Young R. C., Ozols R. F. Augmentation of adriamycin, melphalan, and cisplatin cytotoxicity in drug-resistant and -sensitive human ovarian carcinoma cell lines by buthionine sulfoximine mediated glutathione depletion. Biochem Pharmacol. 1985 Jul 15;34(14):2583–2586. doi: 10.1016/0006-2952(85)90551-9. [DOI] [PubMed] [Google Scholar]
- Hosking L. K., Whelan R. D., Shellard S. A., Bedford P., Hill B. T. An evaluation of the role of glutathione and its associated enzymes in the expression of differential sensitivities to antitumour agents shown by a range of human tumour cell lines. Biochem Pharmacol. 1990 Oct 15;40(8):1833–1842. doi: 10.1016/0006-2952(90)90364-q. [DOI] [PubMed] [Google Scholar]
- Kramer R. A., Greene K., Ahmad S., Vistica D. T. Chemosensitization of L-phenylalanine mustard by the thiol-modulating agent buthionine sulfoximine. Cancer Res. 1987 Mar 15;47(6):1593–1597. [PubMed] [Google Scholar]
- Kramer R. A., Soble M., Howes A. E., Montoya V. P. The effect of glutathione (GSH) depletion in vivo by buthionine sulfoximine (BSO) on the radiosensitization of SR 2508. Int J Radiat Oncol Biol Phys. 1989 May;16(5):1325–1329. doi: 10.1016/0360-3016(89)90308-8. [DOI] [PubMed] [Google Scholar]
- Lee F. Y., Allalunis-Turner M. J., Siemann D. W. Depletion of tumour versus normal tissue glutathione by buthionine sulfoximine. Br J Cancer. 1987 Jul;56(1):33–38. doi: 10.1038/bjc.1987.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F. Y., Flannery D. J., Siemann D. W. Prediction of tumour sensitivity to 4-hydroperoxycyclophosphamide by a glutathione-targeted assay. Br J Cancer. 1991 Feb;63(2):217–222. doi: 10.1038/bjc.1991.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F. Y., Siemann D. W., Allalunis-Turner M. J., Keng P. C. Glutathione contents in human and rodent tumor cells in various phases of the cell cycle. Cancer Res. 1988 Jul 1;48(13):3661–3665. [PubMed] [Google Scholar]
- Lee F. Y., Siemann D. W., Sutherland R. M. Changes in cellular glutathione content during adriamycin treatment in human ovarian cancer--a possible indicator of chemosensitivity. Br J Cancer. 1989 Sep;60(3):291–298. doi: 10.1038/bjc.1989.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A. Selective modification of glutathione metabolism. Science. 1983 Apr 29;220(4596):472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- Minchinton A. I., Rojas A., Smith K. A., Soranson J. A., Shrieve D. C., Jones N. R., Bremner J. C. Glutathione depletion in tissues after administration of buthionine sulphoximine. Int J Radiat Oncol Biol Phys. 1984 Aug;10(8):1261–1264. doi: 10.1016/0360-3016(84)90329-8. [DOI] [PubMed] [Google Scholar]
- Mitchell J. B., Cook J. A., DeGraff W., Glatstein E., Russo A. Glutathione modulation in cancer treatment: will it work? Int J Radiat Oncol Biol Phys. 1989 May;16(5):1289–1295. doi: 10.1016/0360-3016(89)90301-5. [DOI] [PubMed] [Google Scholar]
- Morstyn G., Russo A., Carney D. N., Karawya E., Wilson S. H., Mitchell J. B. Heterogeneity in the radiation survival curves and biochemical properties of human lung cancer cell lines. J Natl Cancer Inst. 1984 Oct;73(4):801–807. [PubMed] [Google Scholar]
- Ono K., Komuro C., Tsutsui K., Shibamoto Y., Nishidai T., Takahashi M., Abe M., Shrieve D. C. Combined effect of buthionine sulfoximine and cyclophosphamide upon murine tumours and bone marrow. Br J Cancer. 1986 Nov;54(5):749–754. doi: 10.1038/bjc.1986.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozols R. F., Louie K. G., Plowman J., Behrens B. C., Fine R. L., Dykes D., Hamilton T. C. Enhanced melphalan cytotoxicity in human ovarian cancer in vitro and in tumor-bearing nude mice by buthionine sulfoximine depletion of glutathione. Biochem Pharmacol. 1987 Jan 1;36(1):147–153. doi: 10.1016/0006-2952(87)90392-3. [DOI] [PubMed] [Google Scholar]
- Ozols R. F. Pharmacologic reversal of drug resistance in ovarian cancer. Semin Oncol. 1985 Sep;12(3 Suppl 4):7–11. [PubMed] [Google Scholar]
- Richardson M. E., Siemann D. W. Thiol manipulation as a means of overcoming drug resistance in a novel cyclophosphamide-induced resistant cell line. Int J Radiat Oncol Biol Phys. 1992;22(4):781–784. doi: 10.1016/0360-3016(92)90523-k. [DOI] [PubMed] [Google Scholar]
- Russo A., Tochner Z., Phillips T., Carmichael J., DeGraff W., Friedman N., Fisher J., Mitchell J. B. In vivo modulation of glutathione by buthionine sulfoximine: effect on marrow response to melphalan. Int J Radiat Oncol Biol Phys. 1986 Jul;12(7):1187–1189. doi: 10.1016/0360-3016(86)90255-5. [DOI] [PubMed] [Google Scholar]
- Suzukake K., Petro B. J., Vistica D. T. Reduction in glutathione content of L-PAM resistant L1210 Cells confers drug sensitivity. Biochem Pharmacol. 1982 Jan 1;31(1):121–124. doi: 10.1016/0006-2952(82)90249-0. [DOI] [PubMed] [Google Scholar]
- Thomson J. E., Rauth A. M. An in vitro assay to measure the viability of KHT tumor cells not previously exposed to culture conditions. Radiat Res. 1974 May;58(2):262–276. [PubMed] [Google Scholar]
- Tsutsui K., Komuro C., Ono K., Nishidai T., Shibamoto Y., Takahashi M., Abe M. Chemosensitization by buthionine sulfoximine in vivo. Int J Radiat Oncol Biol Phys. 1986 Jul;12(7):1183–1186. doi: 10.1016/0360-3016(86)90254-3. [DOI] [PubMed] [Google Scholar]


