Abstract
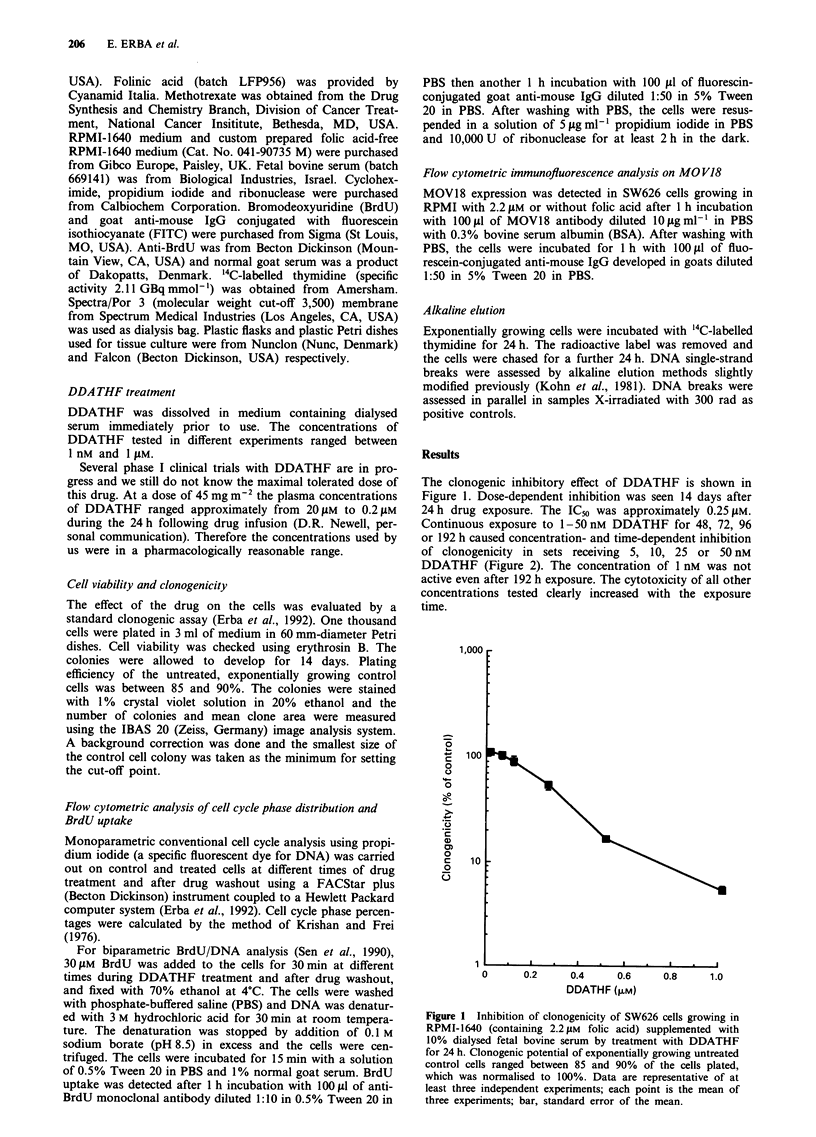
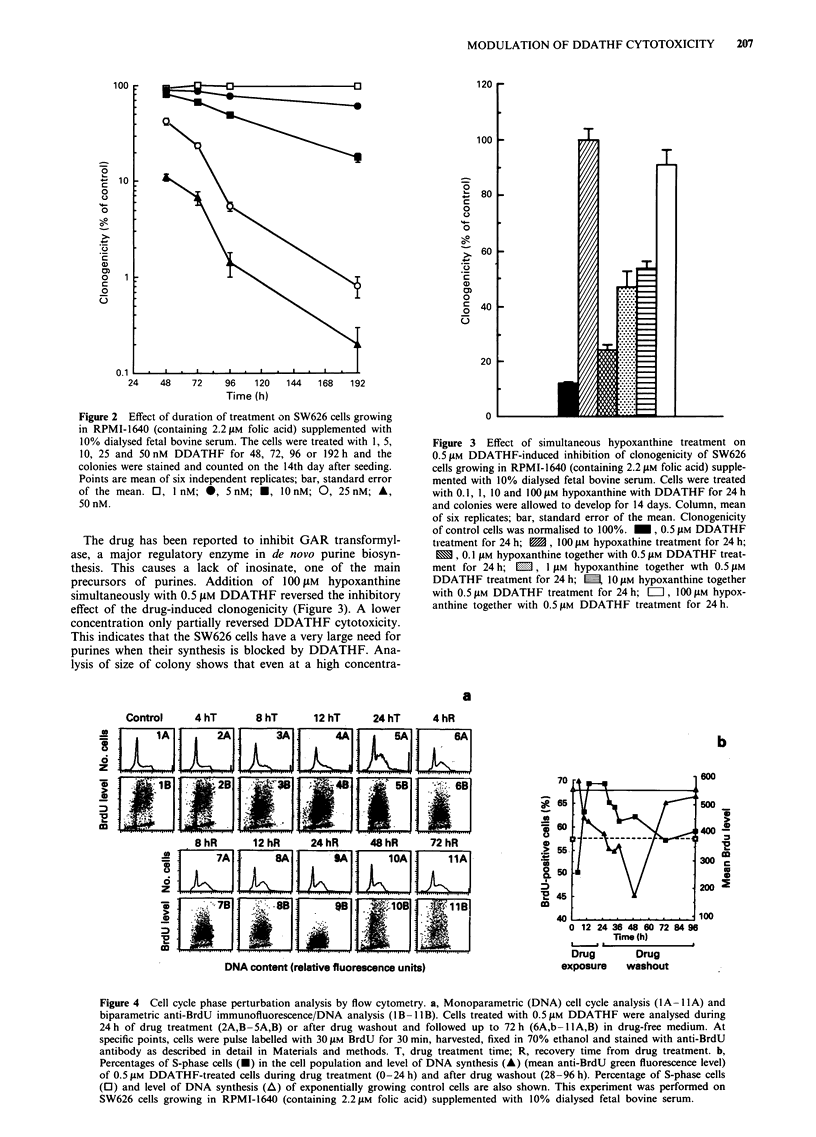
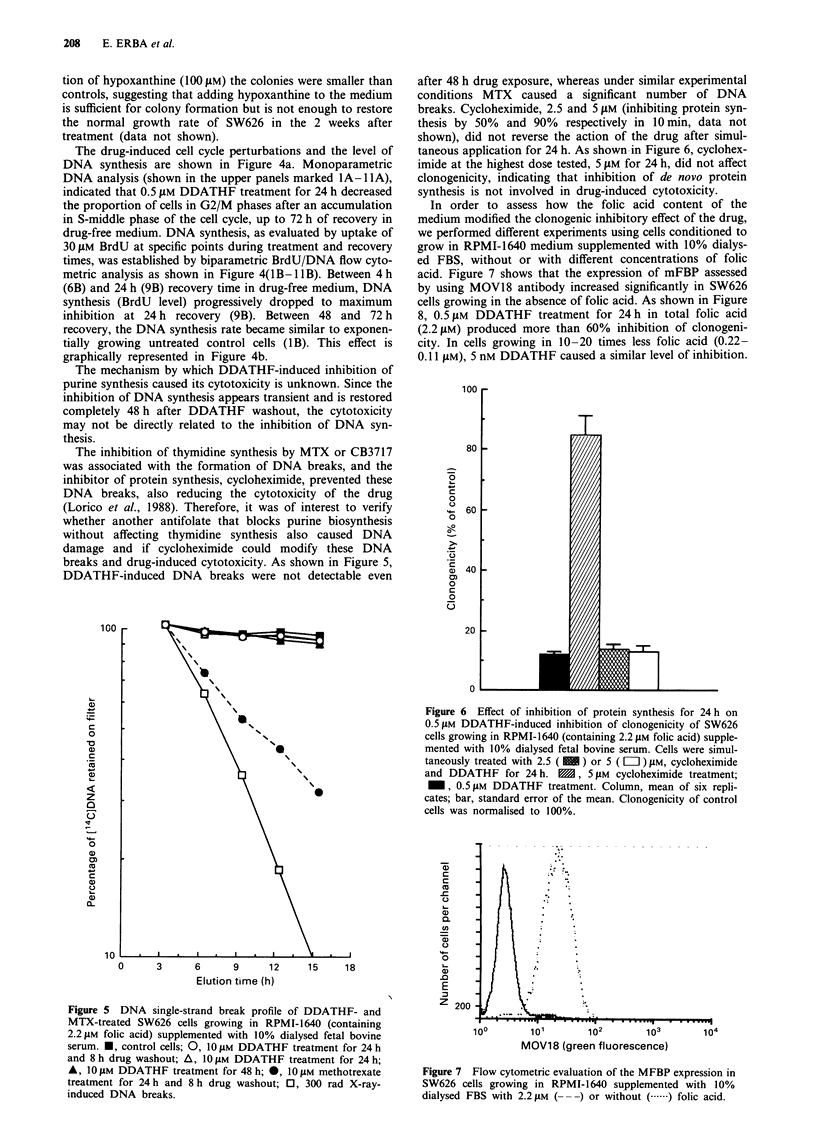
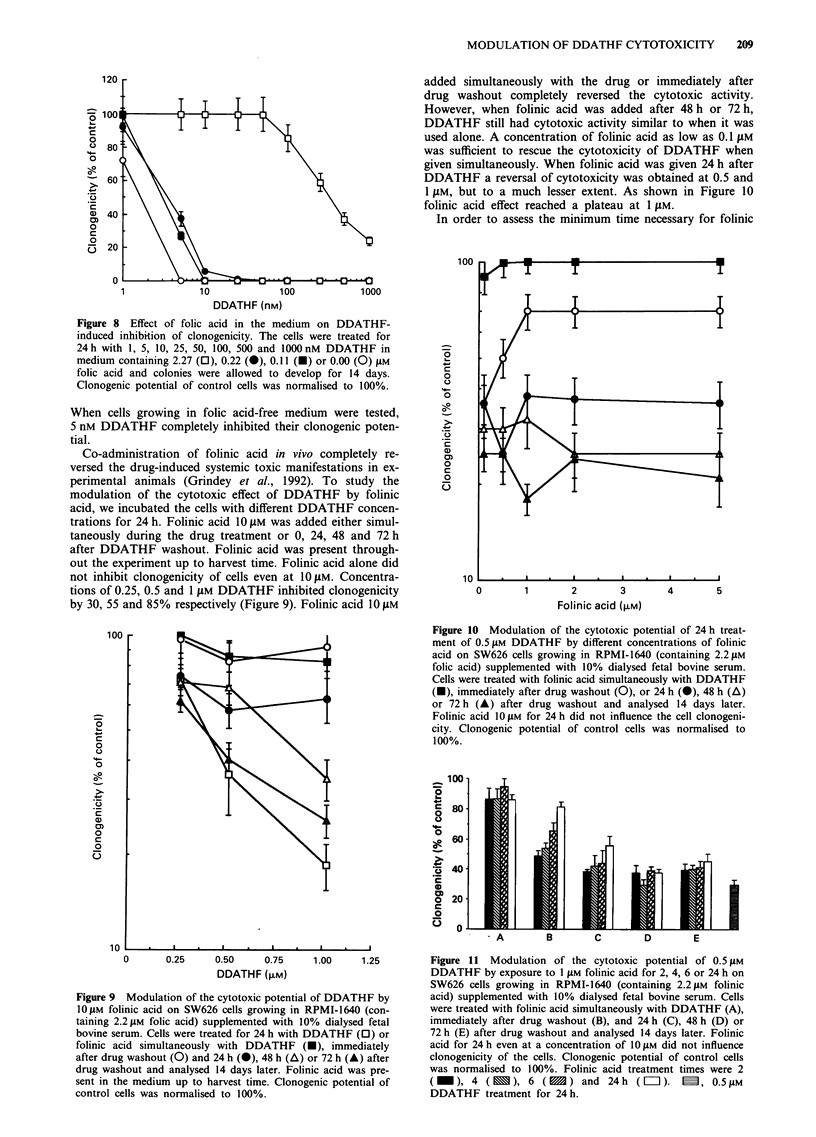
Inhibition of clonogenic potential by the glycinamideribonucleosyl transformylase inhibitor 5,10-dideazatetrahydrofolic acid (DDATHF, Lometrexol) was evaluated in vitro in a human ovarian carcinoma cell line, SW626. Drug-induced inhibition of clonogenic potential is a function of the dose and time of exposure and is independent of the formation of DNA single-strand breaks or de novo synthesis of protein. Simultaneous treatment with 100 microM hypoxanthine completely prevented the inhibition of clonogenic potential caused by 0.5 microM DDATHF. DDATHF blocked cells in the early-middle S-phases of the cell cycle, and there was a corresponding marked reduction in the rate of DNA synthesis after drug withdrawal. The cytotoxic potential of DDATHF was modulated by the folic acid concentration present in the medium. In a medium containing 0.22 microM folic acid, DDATHF cytotoxicity was at least 100 times that in a regular medium containing 2.22 microM folic acid, levels which, however, are about 100 times those found in human plasma. DDATHF cytotoxicity differed moderately when folic acid concentrations varied between 0.22 and 0 microM, suggesting that folic acid does not necessarily antagonise DDATHF anti-tumour activity. Folinic acid at a concentration as low as 0.1 microM can completely rescue cells when given simultaneously with 0.5 microM DDATHF. When folinic acid was given 24 h after DDATHF, a reversal of cytotoxicity was observed at 0.5 and 1 microM, but to a much lesser extent than simultaneous treatment. When folinic acid was added after 48 or 72 h of DDATHF washout, even at a high concentration and for a long time, no reduction in DDATHF cytotoxicity was found. In conclusion, the study highlights the modulation of DDATHF cytotoxicity by folic acid or by folinic acid and provides further rationale for in vivo clinical investigation with these combinations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antony A. C. The biological chemistry of folate receptors. Blood. 1992 Jun 1;79(11):2807–2820. [PubMed] [Google Scholar]
- Baldwin S. W., Tse A., Gossett L. S., Taylor E. C., Rosowsky A., Shih C., Moran R. G. Structural features of 5,10-dideaza-5,6,7,8-tetrahydrofolate that determine inhibition of mammalian glycinamide ribonucleotide formyltransferase. Biochemistry. 1991 Feb 19;30(7):1997–2006. doi: 10.1021/bi00221a037. [DOI] [PubMed] [Google Scholar]
- Beardsley G. P., Moroson B. A., Taylor E. C., Moran R. G. A new folate antimetabolite, 5,10-dideaza-5,6,7,8-tetrahydrofolate is a potent inhibitor of de novo purine synthesis. J Biol Chem. 1989 Jan 5;264(1):328–333. [PubMed] [Google Scholar]
- Borner M., Castiglione M., Triller J., Baer H. U., Soucek M., Blumgart L., Brunner K. Considerable side effects of chemoembolization for colorectal carcinoma metastatic to the liver. Ann Oncol. 1992 Feb;3(2):113–115. doi: 10.1093/oxfordjournals.annonc.a058123. [DOI] [PubMed] [Google Scholar]
- Campbell I. G., Jones T. A., Foulkes W. D., Trowsdale J. Folate-binding protein is a marker for ovarian cancer. Cancer Res. 1991 Oct 1;51(19):5329–5338. [PubMed] [Google Scholar]
- Erba E., Sen S., Lorico A., D'Incalci M. Potentiation of etoposide cytotoxicity against a human ovarian cancer cell line by pretreatment with non-toxic concentrations of methotrexate or aphidicolin. Eur J Cancer. 1992;28(1):66–71. doi: 10.1016/0959-8049(92)90387-h. [DOI] [PubMed] [Google Scholar]
- Jansen G., Kathmann I., Rademaker B. C., Braakhuis B. J., Westerhof G. R., Rijksen G., Schornagel J. H. Expression of a folate binding protein in L1210 cells grown in low folate medium. Cancer Res. 1989 Apr 15;49(8):1959–1963. [PubMed] [Google Scholar]
- Jansen G., Westerhof G. R., Kathmann I., Rijksen G., Schornagel J. H. Growth-inhibitory effects of 5,10-dideazatetrahydrofolic acid on variant murine L1210 and human CCRF-CEM leukemia cells with different membrane-transport characteristics for (anti)folate compounds. Cancer Chemother Pharmacol. 1991;28(2):115–117. doi: 10.1007/BF00689699. [DOI] [PubMed] [Google Scholar]
- Kane M. A., Elwood P. C., Portillo R. M., Antony A. C., Najfeld V., Finley A., Waxman S., Kolhouse J. F. Influence on immunoreactive folate-binding proteins of extracellular folate concentration in cultured human cells. J Clin Invest. 1988 May;81(5):1398–1406. doi: 10.1172/JCI113469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishan A., Frei E., 3rd Effect of adriamycin on the cell cycle traverse and kinetics of cultured human lymphoblasts. Cancer Res. 1976 Jan;36(1):143–150. [PubMed] [Google Scholar]
- Kwok J. B., Tattersall M. H. DNA fragmentation, dATP pool elevation and potentiation of antifolate cytotoxicity in L1210 cells by hypoxanthine. Br J Cancer. 1992 Apr;65(4):503–508. doi: 10.1038/bjc.1992.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. C., Kaminskas E. Accumulation of DNA strand breaks and methotrexate cytotoxicity. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5694–5698. doi: 10.1073/pnas.81.18.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorico A., Toffoli G., Boiocchi M., Erba E., Broggini M., Rappa G., D'Incalci M. Accumulation of DNA strand breaks in cells exposed to methotrexate or N10-propargyl-5,8-dideazafolic acid. Cancer Res. 1988 Apr 15;48(8):2036–2041. [PubMed] [Google Scholar]
- Miotti S., Canevari S., Ménard S., Mezzanzanica D., Porro G., Pupa S. M., Regazzoni M., Tagliabue E., Colnaghi M. I. Characterization of human ovarian carcinoma-associated antigens defined by novel monoclonal antibodies with tumor-restricted specificity. Int J Cancer. 1987 Mar 15;39(3):297–303. doi: 10.1002/ijc.2910390306. [DOI] [PubMed] [Google Scholar]
- Pizzorno G., Cashmore A. R., Moroson B. A., Cross A. D., Smith A. K., Marling-Cason M., Kamen B. A., Beardsley G. P. 5,10-Dideazatetrahydrofolic acid (DDATHF) transport in CCRF-CEM and MA104 cell lines. J Biol Chem. 1993 Jan 15;268(2):1017–1023. [PubMed] [Google Scholar]
- Pizzorno G., Moroson B. A., Cashmore A. R., Beardsley G. P. (6R)-5,10-Dideaza-5,6,7,8-tetrahydrofolic acid effects on nucleotide metabolism in CCRF-CEM human T-lymphoblast leukemia cells. Cancer Res. 1991 May 1;51(9):2291–2295. [PubMed] [Google Scholar]
- Pizzorno G., Sokoloski J. A., Cashmore A. R., Moroson B. A., Cross A. D., Beardsley G. P. Intracellular metabolism of 5,10-dideazatetrahydrofolic acid in human leukemia cell lines. Mol Pharmacol. 1991 Jan;39(1):85–89. [PubMed] [Google Scholar]
- Sen S., Erba E., D'Incalci M. Synchronisation of cancer cell lines of human origin using methotrexate. Cytometry. 1990;11(5):595–602. doi: 10.1002/cyto.990110506. [DOI] [PubMed] [Google Scholar]
- Taylor E. C., Hamby J. M., Shih C., Grindey G. B., Rinzel S. M., Beardsley G. P., Moran R. G. Synthesis and antitumor activity of 5-deaza-5,6,7,8-tetrahydrofolic acid and its N10-substituted analogues. J Med Chem. 1989 Jul;32(7):1517–1522. doi: 10.1021/jm00127a019. [DOI] [PubMed] [Google Scholar]
- Westerhof G. R., Jansen G., van Emmerik N., Kathmann I., Rijksen G., Jackman A. L., Schornagel J. H. Membrane transport of natural folates and antifolate compounds in murine L1210 leukemia cells: role of carrier- and receptor-mediated transport systems. Cancer Res. 1991 Oct 15;51(20):5507–5513. [PubMed] [Google Scholar]


