Abstract
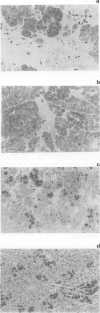
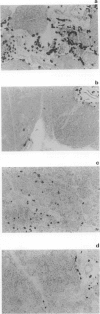
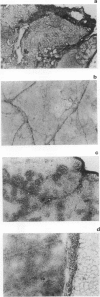
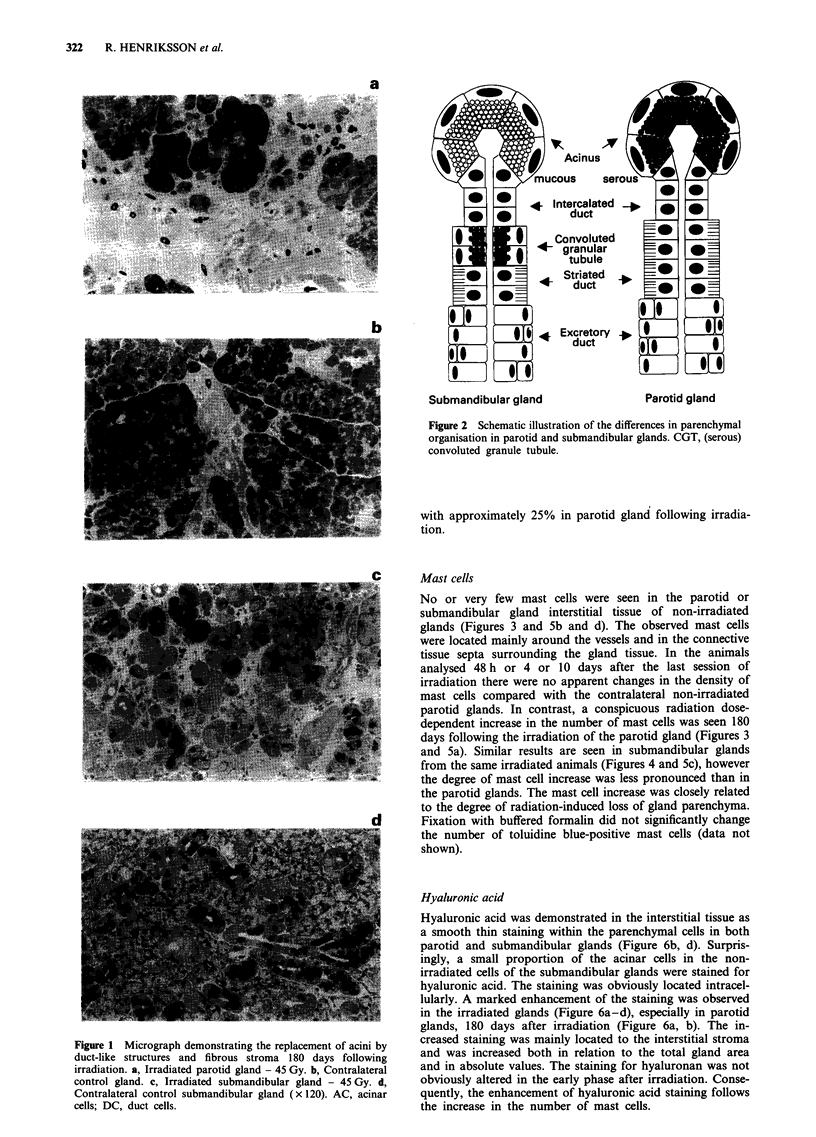
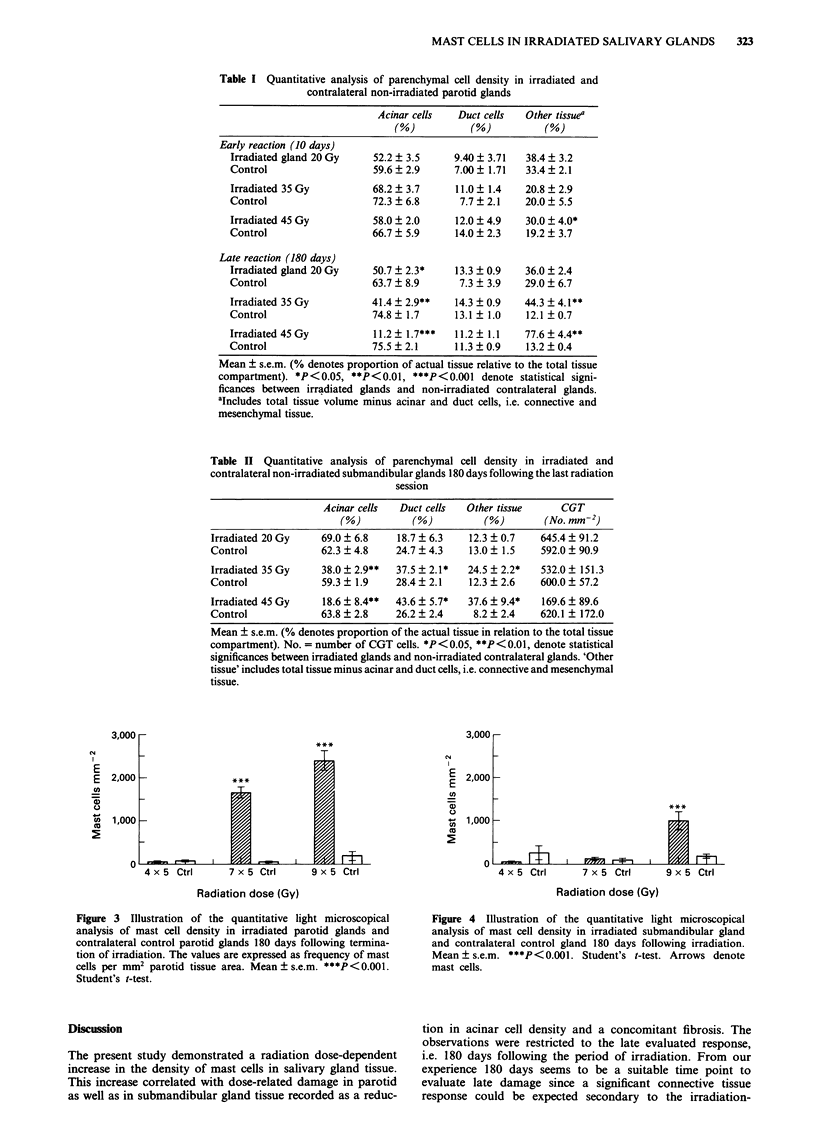
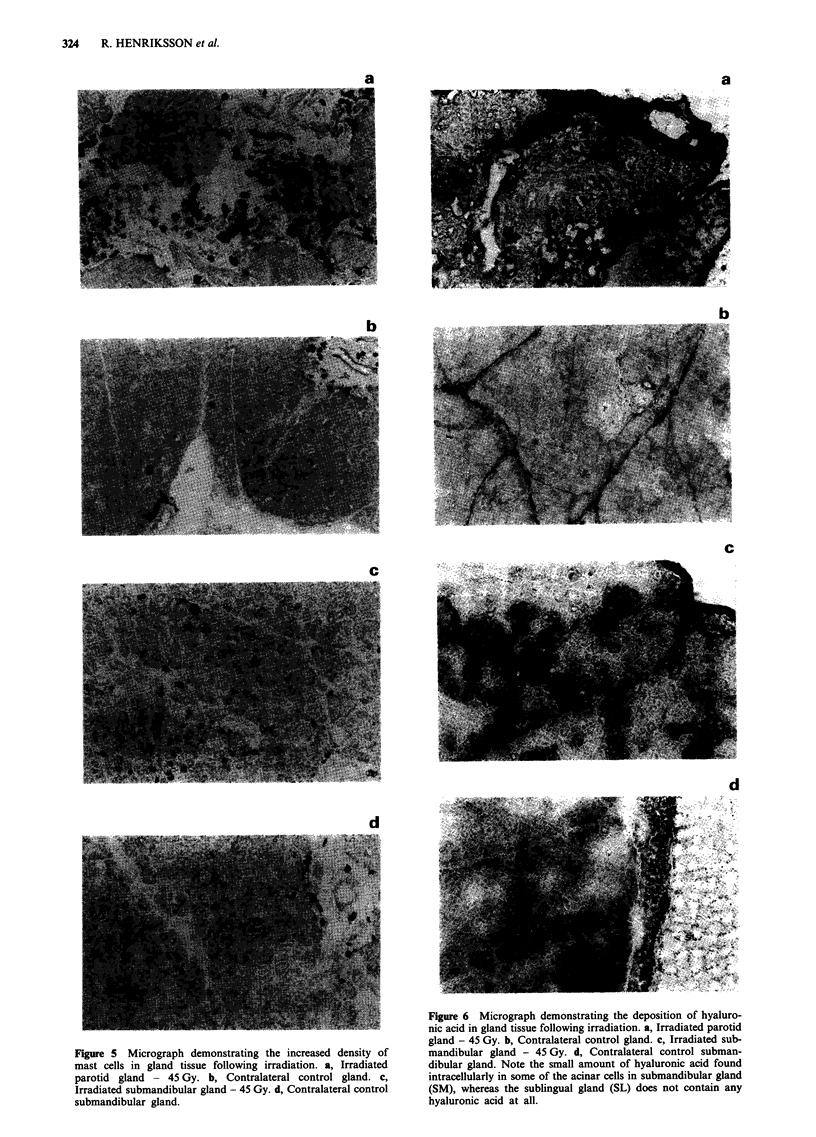
The detailed mechanisms which can explain the inherent radiosensitivity of salivary glands remain to be elucidated. Although DNA is the most plausible critical target for the lethal effects of irradiation, interactions with other constituents, such as cell membrane and neuropeptides, have been suggested to cause important physiological changes. Moreover, mast cells seem to be closely linked to radiation-induced pneumonitis. Therefore, in the present study the effects of fractionated irradiation on salivary glands have been assessed with special regard to the appearance of mast cells and its correlation with damage to gland parenchyma. Sprague-Dawley strain rats were unilaterally irradiated to the head and neck with the salivary glands within the radiation field. The irradiation was delivered once daily for 5 days to a total dose of 20, 35 and 45 Gy. The contralateral parotid and submandibular glands served as intra-animal controls and parallel analysis of glands was performed 2, 4, 10 or 180 days following the last radiation treatment. Morphological analysis revealed no obvious changes up to 10 days after the irradiation. At 180 days a radiation dose-dependent loss of gland parenchyma was seen, especially with regard to serious acinar cells in parotid gland and acinar cells and serous CGT (convoluted granular tubule) cells in the submandibular gland. These changes displayed a close correlation with a concomitant dose-dependent enhanced density of mast cells and staining for hyaluronic acid. This cell population seems to conform with the features of the connective tissue mast cell type. The parotid seems to be more sensitive to irradiation than the submandibular gland. Thus, the present results further strengthen the role of and the potential interaction of mast cells with radiation-induced tissue injury and alterations in normal tissue integrity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abok K., Brunk U., Jung B., Ericsson J. Morphologic and histochemical studies on the differing radiosensitivity of ductular and acinar cells of the rat submandibular gland. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45(4):443–460. doi: 10.1007/BF02889885. [DOI] [PubMed] [Google Scholar]
- Alper T. The role of membrane damage in radiation-induced cell death. Adv Exp Med Biol. 1977;84:139–165. doi: 10.1007/978-1-4684-3279-4_7. [DOI] [PubMed] [Google Scholar]
- Bjermer L., Franzén L., Littbrand B., Nilsson K., Angström T., Henriksson R. Effects of smoking and irradiated volume on inflammatory response in the lung of irradiated breast cancer patients evaluated with bronchoalveolar lavage. Cancer Res. 1990 Apr 1;50(7):2027–2030. [PubMed] [Google Scholar]
- Bjermer L., Hällgren R., Nilsson K., Franzen L., Sandström T., Särnstrand B., Henriksson R. Radiation-induced increase in hyaluronan and fibronectin in bronchoalveolar lavage fluid from breast cancer patients is suppressed by smoking. Eur Respir J. 1992 Jul;5(7):785–790. [PubMed] [Google Scholar]
- CREASEY W. A. Changes in the sodium and potassium contents of cells nuclei after irradiation. Biochim Biophys Acta. 1960 Feb 12;38:181–182. doi: 10.1016/0006-3002(60)91221-x. [DOI] [PubMed] [Google Scholar]
- DESAI I. D., SAWANT P. L., TAPPEL A. L. PEROXIDATIVE AND RADIATION DAMAGE TO ISOLATED LYSOSOMES. Biochim Biophys Acta. 1964 May 11;86:277–285. doi: 10.1016/0304-4165(64)90054-6. [DOI] [PubMed] [Google Scholar]
- El-Mofty S. K., Kahn A. J. Early membrane injury in lethally irradiated salivary gland cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1981 Jan;39(1):55–62. doi: 10.1080/09553008114550071. [DOI] [PubMed] [Google Scholar]
- Enerbäck L. Mast cells in rat gastrointestinal mucosa. 2. Dye-binding and metachromatic properties. Acta Pathol Microbiol Scand. 1966;66(3):303–312. doi: 10.1111/apm.1966.66.3.303. [DOI] [PubMed] [Google Scholar]
- Eneroth C. M., Henrikson C. O., Jakobsson P. A. Effect of fractionated radiotherapy on salivary gland function. Cancer. 1972 Nov;30(5):1147–1153. doi: 10.1002/1097-0142(197211)30:5<1147::aid-cncr2820300502>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Forsgren S., Franzén L., Funegård U., Gustafsson H., Henriksson R. Bilateral irradiation of head and neck induces an enhanced expression of substance P in the parasympathetic innervation of the submandibular gland. Neuroscience. 1992;46(1):233–240. doi: 10.1016/0306-4522(92)90023-u. [DOI] [PubMed] [Google Scholar]
- Franzén L., Bjermer L., Henriksson R., Littbrand B., Nilsson K. Does smoking protect against radiation-induced pneumonitis? Int J Radiat Biol. 1989 Nov;56(5):721–724. doi: 10.1080/09553008914551961. [DOI] [PubMed] [Google Scholar]
- Franzén L., Forsgren S., Gustafsson H., Henriksson R. Irradiation-induced effects on the innervation of rat salivary glands: changes in enkephalin- and bombesin-like immunoreactivity in ganglionic cells and intraglandular nerve fibers. Cell Tissue Res. 1993 Mar;271(3):529–536. doi: 10.1007/BF02913737. [DOI] [PubMed] [Google Scholar]
- Franzén L., Funegård U., Sundström S., Gustafsson H., Danielsson A., Henriksson R. Fractionated irradiation and early changes in salivary glands. Different effects on potassium efflux, exocytotic amylase release and gland morphology. Lab Invest. 1991 Feb;64(2):279–283. [PubMed] [Google Scholar]
- Graham M. M., Evans M. L., Dahlen D. D., Mahler P. A., Rasey J. S. Pharmacological alteration of the lung vascular response to radiation. Int J Radiat Oncol Biol Phys. 1990 Aug;19(2):329–339. doi: 10.1016/0360-3016(90)90541-q. [DOI] [PubMed] [Google Scholar]
- Henriksson R. beta 1- and beta 2-adrenoceptor agonists have different effects on rat parotid acinar cells. Am J Physiol. 1982 May;242(5):G481–G485. doi: 10.1152/ajpgi.1982.242.5.G481. [DOI] [PubMed] [Google Scholar]
- Junglee D., Katrak A., Mohiuddin J., Blacklock H., Prentice H. G., Dandona P. Salivary amylase and pancreatic enzymes in serum after total body irradiation. Clin Chem. 1986 Apr;32(4):609–610. [PubMed] [Google Scholar]
- Mira J. G., Fullerton G. D., Wescott W. B. Correlation between initial salivary flow rate and radiation dose in the production of xerostomia. Acta Radiol Oncol. 1982;21(3):151–154. doi: 10.3109/02841868209133999. [DOI] [PubMed] [Google Scholar]
- Nettelbladt O., Bergh J., Schenholm M., Tengblad A., Hällgren R. Accumulation of hyaluronic acid in the alveolar interstitial tissue in bleomycin-induced alveolitis. Am Rev Respir Dis. 1989 Mar;139(3):759–762. doi: 10.1164/ajrccm/139.3.759. [DOI] [PubMed] [Google Scholar]
- Nilsson K., Bjermer L., Hellström S., Henriksson R., Hällgren R. A mast cell secretagogue, compound 48/80, prevents the accumulation of hyaluronan in lung tissue injured by ionizing irradiation. Am J Respir Cell Mol Biol. 1990 Feb;2(2):199–205. doi: 10.1165/ajrcmb/2.2.199. [DOI] [PubMed] [Google Scholar]
- Nilsson K., Henriksson R., Hellström S., Tengblad A., Bjermer L. Hyaluronan reflects the pre-fibrotic inflammation in irradiated rat lung: concomitant analysis of parenchymal tissues and bronchoalveolar lavage. Int J Radiat Biol. 1990 Sep;58(3):519–530. doi: 10.1080/09553009014551861. [DOI] [PubMed] [Google Scholar]
- Reade P. C., Steidler N. E. X-irradiation induced degranulation of cells of convoluted granular tubules of murine submandibular salivary glands. J Biol Buccale. 1985 Dec;13(4):307–315. [PubMed] [Google Scholar]
- Shannon I. L., Trodahl J. N., Starcke E. N. Radiosensitivity of the human parotid gland. Proc Soc Exp Biol Med. 1978 Jan;157(1):50–53. doi: 10.3181/00379727-157-39988. [DOI] [PubMed] [Google Scholar]
- Stephens L. C., Ang K. K., Schultheiss T. E., King G. K., Brock W. A., Peters L. J. Target cell and mode of radiation injury in rhesus salivary glands. Radiother Oncol. 1986 Oct;7(2):165–174. doi: 10.1016/s0167-8140(86)80096-2. [DOI] [PubMed] [Google Scholar]
- Stephens L. C., King G. K., Peters L. J., Ang K. K., Schultheiss T. E., Jardine J. H. Acute and late radiation injury in rhesus monkey parotid glands. Evidence of interphase cell death. Am J Pathol. 1986 Sep;124(3):469–478. [PMC free article] [PubMed] [Google Scholar]
- Sutherland R. M., Stannard J. N., Weed R. I. Involvement of sulphhydryl groups in radiation damage to the human erythrocyte membrane. Int J Radiat Biol Relat Stud Phys Chem Med. 1967;12(6):551–564. doi: 10.1080/09553006714551161. [DOI] [PubMed] [Google Scholar]