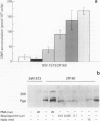
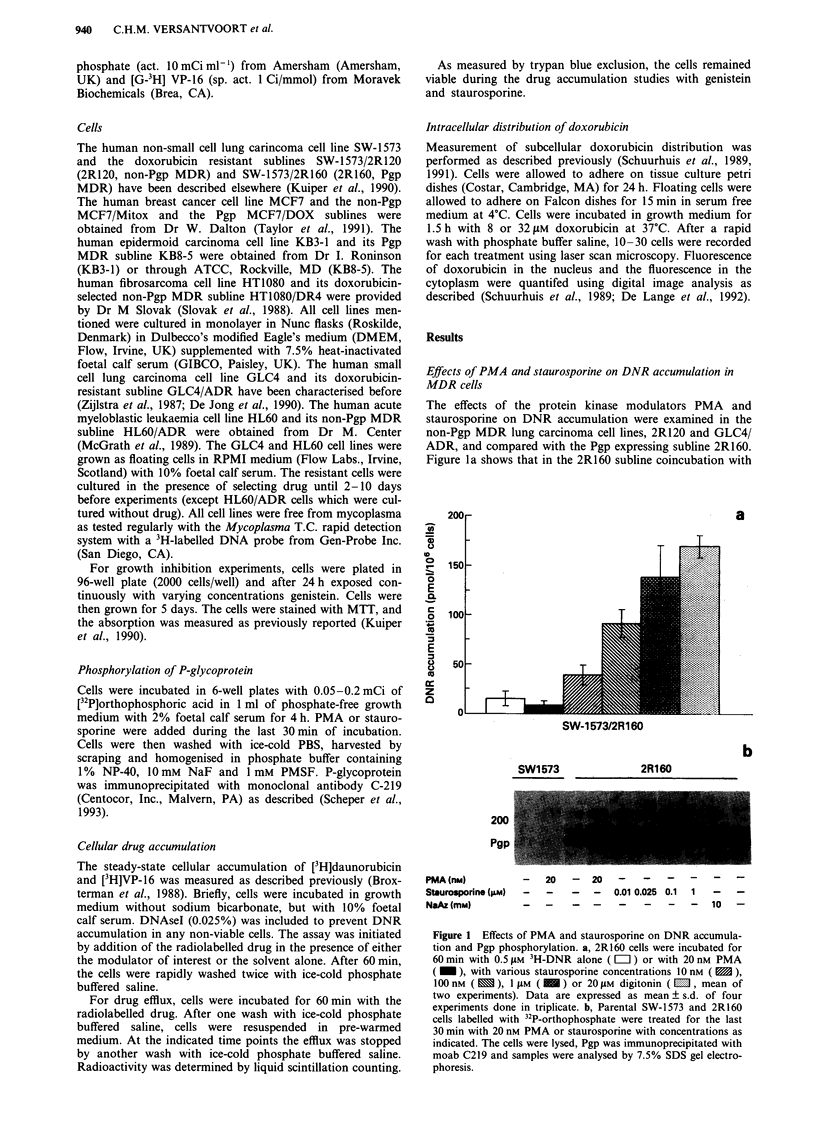
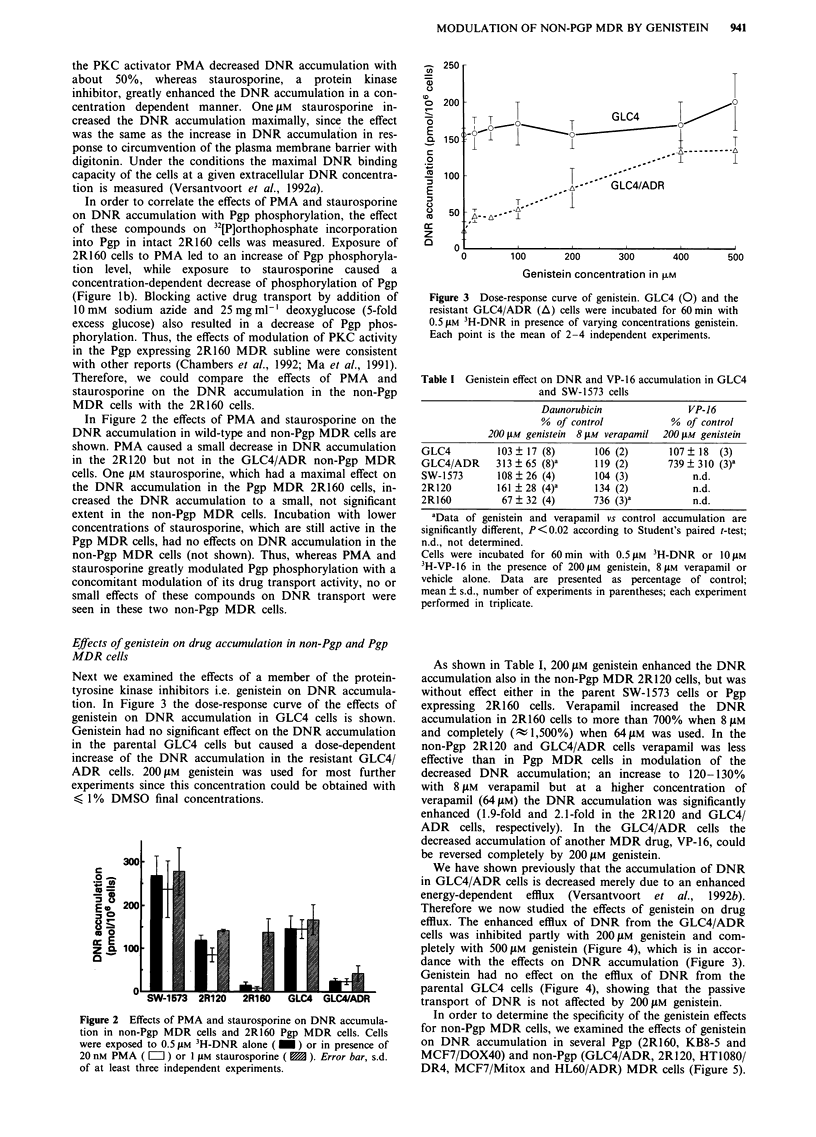
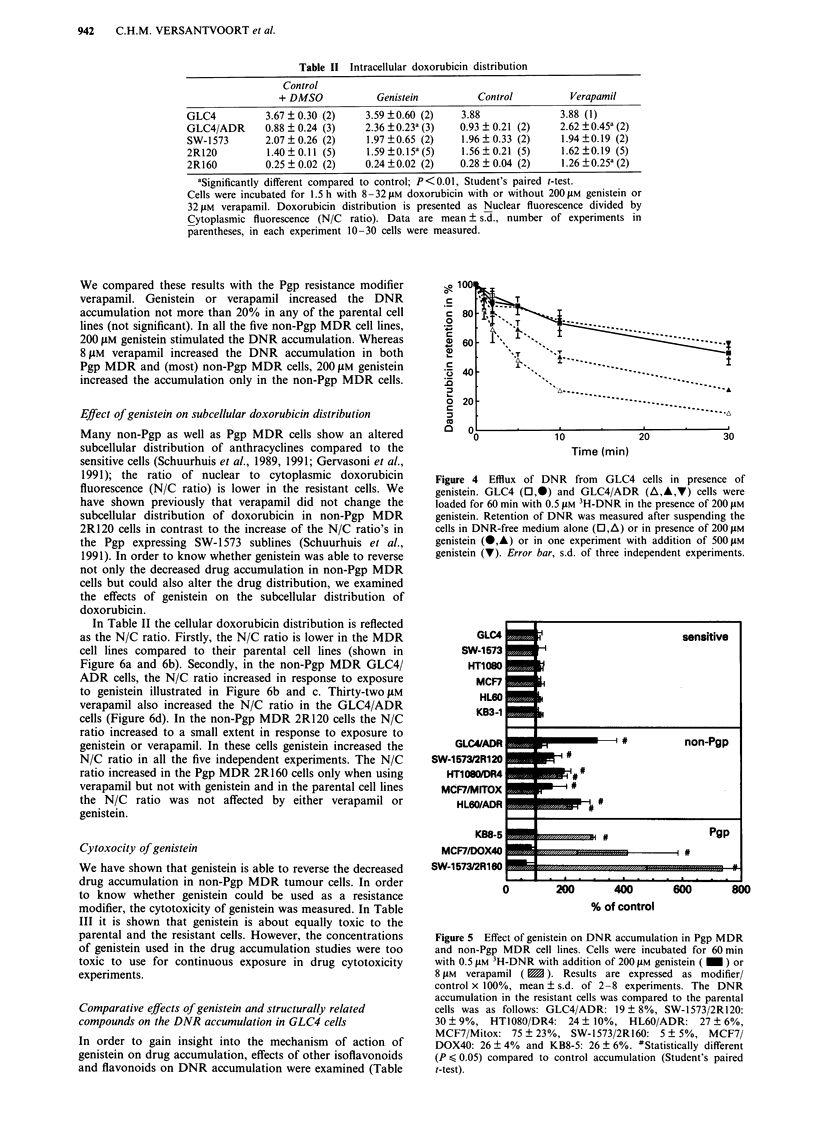
Abstract
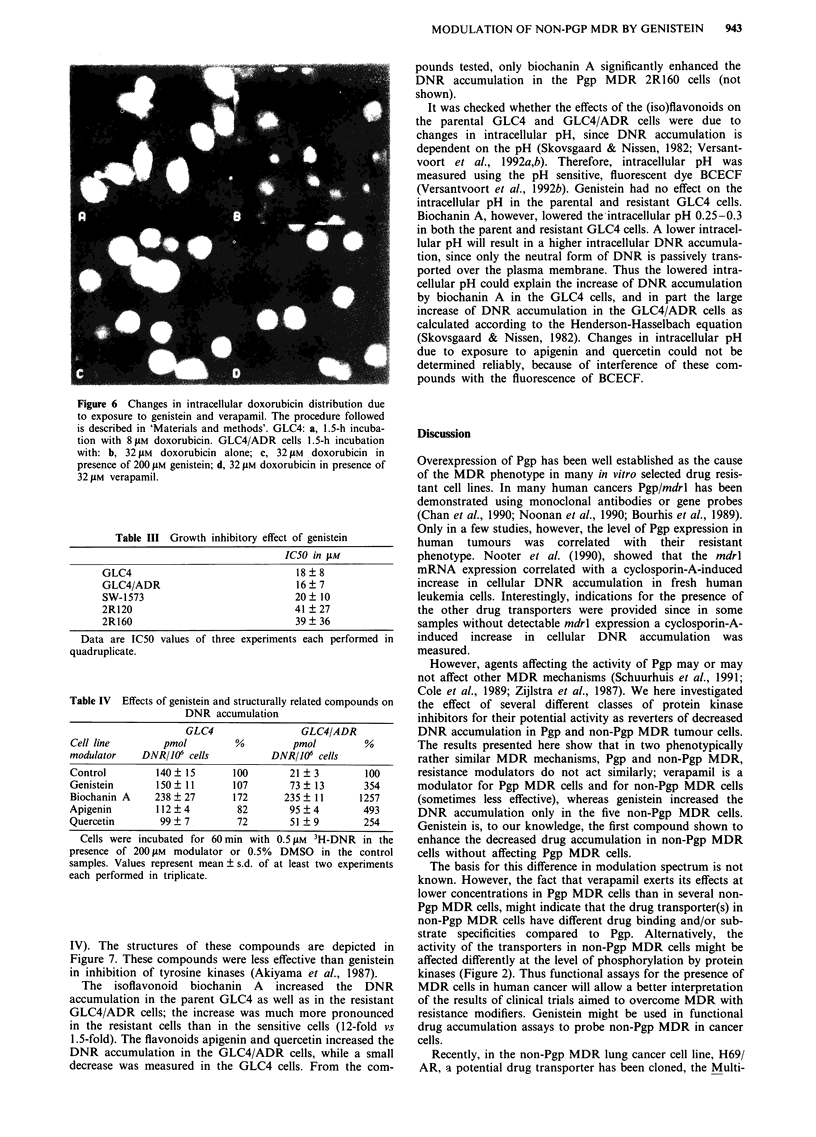
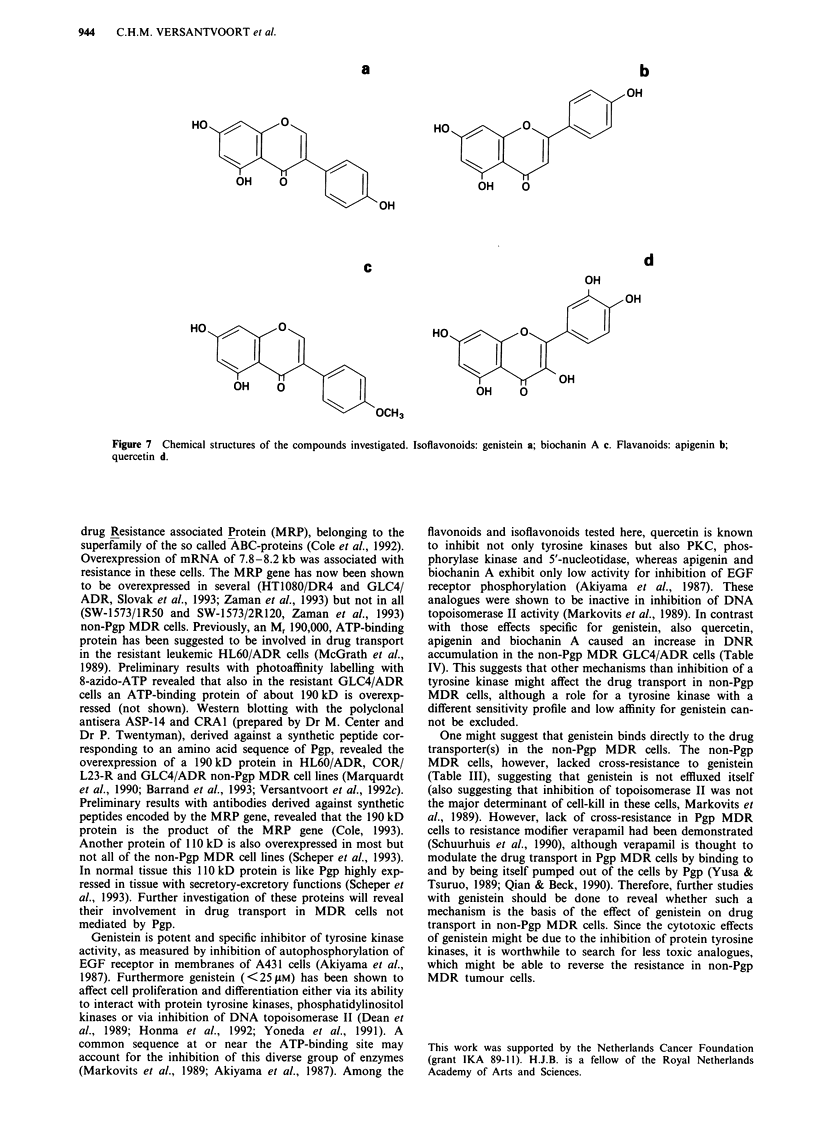
In tumour cells the pharmacological basis for multidrug resistance (MDR) often appears to be a reduced cellular cytostatic drug accumulation caused by the drug efflux protein, P-glycoprotein (Pgp MDR), or by other drug transporters (non-Pgp MDR). Here we report the reversal of the decreased daunorubicin (DNR) accumulation in five non-Pgp MDR cell lines (GLC4/ADR, SW-1573/2R120, HT1080/DR4, MCF7/Mitox and HL60/ADR) by genistein. Genistein inhibited the enhanced DNR efflux in the GLC4/ADR cells. In these cells the decreased VP-16 accumulation was also reversed by genistein. Three other (iso)flavonoids biochanin A, apigenin and quercetin also increased the DNR accumulation in the GLC4/ADR cells. In contrast to the effects on non-Pgp MDR cells, 200 microM genistein did not increase the reduced DNR accumulation in three Pgp MDR cell lines (SW-1573/2R160, MCF7/DOX40 and KB8-5) or in the parental cell lines. In conclusion the use of genistein provides a means to probe non-Pgp related drug accumulation defects.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Baas F., Jongsma A. P., Broxterman H. J., Arceci R. J., Housman D., Scheffer G. L., Riethorst A., van Groenigen M., Nieuwint A. W., Joenje H. Non-P-glycoprotein mediated mechanism for multidrug resistance precedes P-glycoprotein expression during in vitro selection for doxorubicin resistance in a human lung cancer cell line. Cancer Res. 1990 Sep 1;50(17):5392–5398. [PubMed] [Google Scholar]
- Barrand M. A., Rhodes T., Center M. S., Twentyman P. R. Chemosensitisation and drug accumulation effects of cyclosporin A, PSC-833 and verapamil in human MDR large cell lung cancer cells expressing a 190k membrane protein distinct from P-glycoprotein. Eur J Cancer. 1993;29A(3):408–415. doi: 10.1016/0959-8049(93)90397-x. [DOI] [PubMed] [Google Scholar]
- Bates S. E., Currier S. J., Alvarez M., Fojo A. T. Modulation of P-glycoprotein phosphorylation and drug transport by sodium butyrate. Biochemistry. 1992 Jul 21;31(28):6366–6372. doi: 10.1021/bi00143a002. [DOI] [PubMed] [Google Scholar]
- Bourhis J., Bénard J., Hartmann O., Boccon-Gibod L., Lemerle J., Riou G. Correlation of MDR1 gene expression with chemotherapy in neuroblastoma. J Natl Cancer Inst. 1989 Sep 20;81(18):1401–1405. doi: 10.1093/jnci/81.18.1401. [DOI] [PubMed] [Google Scholar]
- Broxterman H. J., Kuiper C. M., Schuurhuis G. J., Tsuruo T., Pinedo H. M., Lankelma J. Increase of daunorubicin and vincristine accumulation in multidrug resistant human ovarian carcinoma cells by a monoclonal antibody reacting with P-glycoprotein. Biochem Pharmacol. 1988 Jun 15;37(12):2389–2393. doi: 10.1016/0006-2952(88)90365-6. [DOI] [PubMed] [Google Scholar]
- Chambers T. C., Zheng B., Kuo J. F. Regulation by phorbol ester and protein kinase C inhibitors, and by a protein phosphatase inhibitor (okadaic acid), of P-glycoprotein phosphorylation and relationship to drug accumulation in multidrug-resistant human KB cells. Mol Pharmacol. 1992 Jun;41(6):1008–1015. [PubMed] [Google Scholar]
- Chan H. S., Thorner P. S., Haddad G., Ling V. Immunohistochemical detection of P-glycoprotein: prognostic correlation in soft tissue sarcoma of childhood. J Clin Oncol. 1990 Apr;8(4):689–704. doi: 10.1200/JCO.1990.8.4.689. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Bhardwaj G., Gerlach J. H., Mackie J. E., Grant C. E., Almquist K. C., Stewart A. J., Kurz E. U., Duncan A. M., Deeley R. G. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992 Dec 4;258(5088):1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Downes H. F., Slovak M. L. Effect of calcium antagonists on the chemosensitivity of two multidrug-resistant human tumour cell lines which do not overexpress P-glycoprotein. Br J Cancer. 1989 Jan;59(1):42–46. doi: 10.1038/bjc.1989.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coley H. M., Workman P., Twentyman P. R. Retention of activity by selected anthracyclines in a multidrug resistant human large cell lung carcinoma line without P-glycoprotein hyperexpression. Br J Cancer. 1991 Mar;63(3):351–357. doi: 10.1038/bjc.1991.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean N. M., Kanemitsu M., Boynton A. L. Effects of the tyrosine-kinase inhibitor genistein on DNA synthesis and phospholipid-derived second messenger generation in mouse 10T1/2 fibroblasts and rat liver T51B cells. Biochem Biophys Res Commun. 1989 Dec 15;165(2):795–801. doi: 10.1016/s0006-291x(89)80036-1. [DOI] [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Fine R. L., Patel J., Chabner B. A. Phorbol esters induce multidrug resistance in human breast cancer cells. Proc Natl Acad Sci U S A. 1988 Jan;85(2):582–586. doi: 10.1073/pnas.85.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasoni J. E., Jr, Fields S. Z., Krishna S., Baker M. A., Rosado M., Thuraisamy K., Hindenburg A. A., Taub R. N. Subcellular distribution of daunorubicin in P-glycoprotein-positive and -negative drug-resistant cell lines using laser-assisted confocal microscopy. Cancer Res. 1991 Sep 15;51(18):4955–4963. [PubMed] [Google Scholar]
- Honma Y., Okabe-Kado J., Kasukabe T., Hozumi M., Kodama H., Kajigaya S., Suda T., Miura Y. Herbimycin A, an inhibitor of tyrosine kinase, prolongs survival of mice inoculated with myeloid leukemia C1 cells with high expression of v-abl tyrosine kinase. Cancer Res. 1992 Jul 15;52(14):4017–4020. [PubMed] [Google Scholar]
- Lai S. L., Goldstein L. J., Gottesman M. M., Pastan I., Tsai C. M., Johnson B. E., Mulshine J. L., Ihde D. C., Kayser K., Gazdar A. F. MDR1 gene expression in lung cancer. J Natl Cancer Inst. 1989 Aug 2;81(15):1144–1150. doi: 10.1093/jnci/81.15.1144. [DOI] [PubMed] [Google Scholar]
- Ma L. D., Marquardt D., Takemoto L., Center M. S. Analysis of P-glycoprotein phosphorylation in HL60 cells isolated for resistance to vincristine. J Biol Chem. 1991 Mar 25;266(9):5593–5599. [PubMed] [Google Scholar]
- Markovits J., Linassier C., Fossé P., Couprie J., Pierre J., Jacquemin-Sablon A., Saucier J. M., Le Pecq J. B., Larsen A. K. Inhibitory effects of the tyrosine kinase inhibitor genistein on mammalian DNA topoisomerase II. Cancer Res. 1989 Sep 15;49(18):5111–5117. [PubMed] [Google Scholar]
- Marquardt D., McCrone S., Center M. S. Mechanisms of multidrug resistance in HL60 cells: detection of resistance-associated proteins with antibodies against synthetic peptides that correspond to the deduced sequence of P-glycoprotein. Cancer Res. 1990 Mar 1;50(5):1426–1430. [PubMed] [Google Scholar]
- McGrath T., Latoud C., Arnold S. T., Safa A. R., Felsted R. L., Center M. S. Mechanisms of multidrug resistance in HL60 cells. Analysis of resistance associated membrane proteins and levels of mdr gene expression. Biochem Pharmacol. 1989 Oct 15;38(20):3611–3619. doi: 10.1016/0006-2952(89)90134-2. [DOI] [PubMed] [Google Scholar]
- Noonan K. E., Beck C., Holzmayer T. A., Chin J. E., Wunder J. S., Andrulis I. L., Gazdar A. F., Willman C. L., Griffith B., Von Hoff D. D. Quantitative analysis of MDR1 (multidrug resistance) gene expression in human tumors by polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7160–7164. doi: 10.1073/pnas.87.18.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooter K., Sonneveld P., Oostrum R., Herweijer H., Hagenbeek T., Valerio D. Overexpression of the mdr1 gene in blast cells from patients with acute myelocytic leukemia is associated with decreased anthracycline accumulation that can be restored by cyclosporin-A. Int J Cancer. 1990 Feb 15;45(2):263–268. doi: 10.1002/ijc.2910450210. [DOI] [PubMed] [Google Scholar]
- Qian X. D., Beck W. T. Binding of an optically pure photoaffinity analogue of verapamil, LU-49888, to P-glycoprotein from multidrug-resistant human leukemic cell lines. Cancer Res. 1990 Feb 15;50(4):1132–1137. [PubMed] [Google Scholar]
- Scheper R. J., Broxterman H. J., Scheffer G. L., Kaaijk P., Dalton W. S., van Heijningen T. H., van Kalken C. K., Slovak M. L., de Vries E. G., van der Valk P. Overexpression of a M(r) 110,000 vesicular protein in non-P-glycoprotein-mediated multidrug resistance. Cancer Res. 1993 Apr 1;53(7):1475–1479. [PubMed] [Google Scholar]
- Schuurhuis G. J., Broxterman H. J., Cervantes A., van Heijningen T. H., de Lange J. H., Baak J. P., Pinedo H. M., Lankelma J. Quantitative determination of factors contributing to doxorubicin resistance in multidrug-resistant cells. J Natl Cancer Inst. 1989 Dec 20;81(24):1887–1892. doi: 10.1093/jnci/81.24.1887. [DOI] [PubMed] [Google Scholar]
- Schuurhuis G. J., Broxterman H. J., de Lange J. H., Pinedo H. M., van Heijningen T. H., Kuiper C. M., Scheffer G. L., Scheper R. J., van Kalken C. K., Baak J. P. Early multidrug resistance, defined by changes in intracellular doxorubicin distribution, independent of P-glycoprotein. Br J Cancer. 1991 Nov;64(5):857–861. doi: 10.1038/bjc.1991.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurhuis G. J., Pinedo H. M., Broxterman H. J., van Kalken C. K., Kuiper C. M., Lankelma J. Differential sensitivity of multi-drug-resistant and -sensitive cells to resistance-modifying agents and the relation with reversal of anthracycline resistance. Int J Cancer. 1990 Aug 15;46(2):330–336. doi: 10.1002/ijc.2910460232. [DOI] [PubMed] [Google Scholar]
- Skovsgaard T., Nissen N. I. Membrane transport of anthracyclines. Pharmacol Ther. 1982;18(3):293–311. doi: 10.1016/0163-7258(82)90034-1. [DOI] [PubMed] [Google Scholar]
- Slovak M. L., Hoeltge G. A., Dalton W. S., Trent J. M. Pharmacological and biological evidence for differing mechanisms of doxorubicin resistance in two human tumor cell lines. Cancer Res. 1988 May 15;48(10):2793–2797. [PubMed] [Google Scholar]
- Takeda Y., Nishio K., Sugimoto Y., Kasahara K., Kubo S., Fujiwara Y., Niitani H., Saijo N. Establishment of a human leukemia subline resistant to the growth-inhibitory effect of 12-O-tetradecanoylphorbol 13-acetate (TPA) and showing non-P-glycoprotein-mediated multi-drug resistance. Int J Cancer. 1991 Jul 30;48(6):931–937. doi: 10.1002/ijc.2910480622. [DOI] [PubMed] [Google Scholar]
- Taylor C. W., Dalton W. S., Parrish P. R., Gleason M. C., Bellamy W. T., Thompson F. H., Roe D. J., Trent J. M. Different mechanisms of decreased drug accumulation in doxorubicin and mitoxantrone resistant variants of the MCF7 human breast cancer cell line. Br J Cancer. 1991 Jun;63(6):923–929. doi: 10.1038/bjc.1991.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versantvoort C. H., Broxterman H. J., Feller N., Dekker H., Kuiper C. M., Lankelma J. Probing daunorubicin accumulation defects in non-P-glycoprotein expressing multidrug-resistant cell lines using digitonin. Int J Cancer. 1992 Apr 1;50(6):906–911. doi: 10.1002/ijc.2910500615. [DOI] [PubMed] [Google Scholar]
- Versantvoort C. H., Broxterman H. J., Pinedo H. M., de Vries E. G., Feller N., Kuiper C. M., Lankelma J. Energy-dependent processes involved in reduced drug accumulation in multidrug-resistant human lung cancer cell lines without P-glycoprotein expression. Cancer Res. 1992 Jan 1;52(1):17–23. [PubMed] [Google Scholar]
- Yoneda T., Lyall R. M., Alsina M. M., Persons P. E., Spada A. P., Levitzki A., Zilberstein A., Mundy G. R. The antiproliferative effects of tyrosine kinase inhibitors tyrphostins on a human squamous cell carcinoma in vitro and in nude mice. Cancer Res. 1991 Aug 15;51(16):4430–4435. [PubMed] [Google Scholar]
- Yu G., Ahmad S., Aquino A., Fairchild C. R., Trepel J. B., Ohno S., Suzuki K., Tsuruo T., Cowan K. H., Glazer R. I. Transfection with protein kinase C alpha confers increased multidrug resistance to MCF-7 cells expressing P-glycoprotein. Cancer Commun. 1991 Jun;3(6):181–189. doi: 10.3727/095535491820873263. [DOI] [PubMed] [Google Scholar]
- Yusa K., Tsuruo T. Reversal mechanism of multidrug resistance by verapamil: direct binding of verapamil to P-glycoprotein on specific sites and transport of verapamil outward across the plasma membrane of K562/ADM cells. Cancer Res. 1989 Sep 15;49(18):5002–5006. [PubMed] [Google Scholar]
- Zaman G. J., Versantvoort C. H., Smit J. J., Eijdems E. W., de Haas M., Smith A. J., Broxterman H. J., Mulder N. H., de Vries E. G., Baas F. Analysis of the expression of MRP, the gene for a new putative transmembrane drug transporter, in human multidrug resistant lung cancer cell lines. Cancer Res. 1993 Apr 15;53(8):1747–1750. [PubMed] [Google Scholar]
- Zijlstra J. G., de Vries E. G., Mulder N. H. Multifactorial drug resistance in an adriamycin-resistant human small cell lung carcinoma cell line. Cancer Res. 1987 Apr 1;47(7):1780–1784. [PubMed] [Google Scholar]
- de Jong S., Zijlstra J. G., de Vries E. G., Mulder N. H. Reduced DNA topoisomerase II activity and drug-induced DNA cleavage activity in an adriamycin-resistant human small cell lung carcinoma cell line. Cancer Res. 1990 Jan 15;50(2):304–309. [PubMed] [Google Scholar]
- de Lange J. H., Schipper N. W., Schuurhuis G. J., ten Kate T. K., van Heijningen T. H., Pinedo H. M., Lankelma J., Baak J. P. Quantification by laser scan microscopy of intracellular doxorubicin distribution. Cytometry. 1992;13(6):571–576. doi: 10.1002/cyto.990130604. [DOI] [PubMed] [Google Scholar]