Abstract
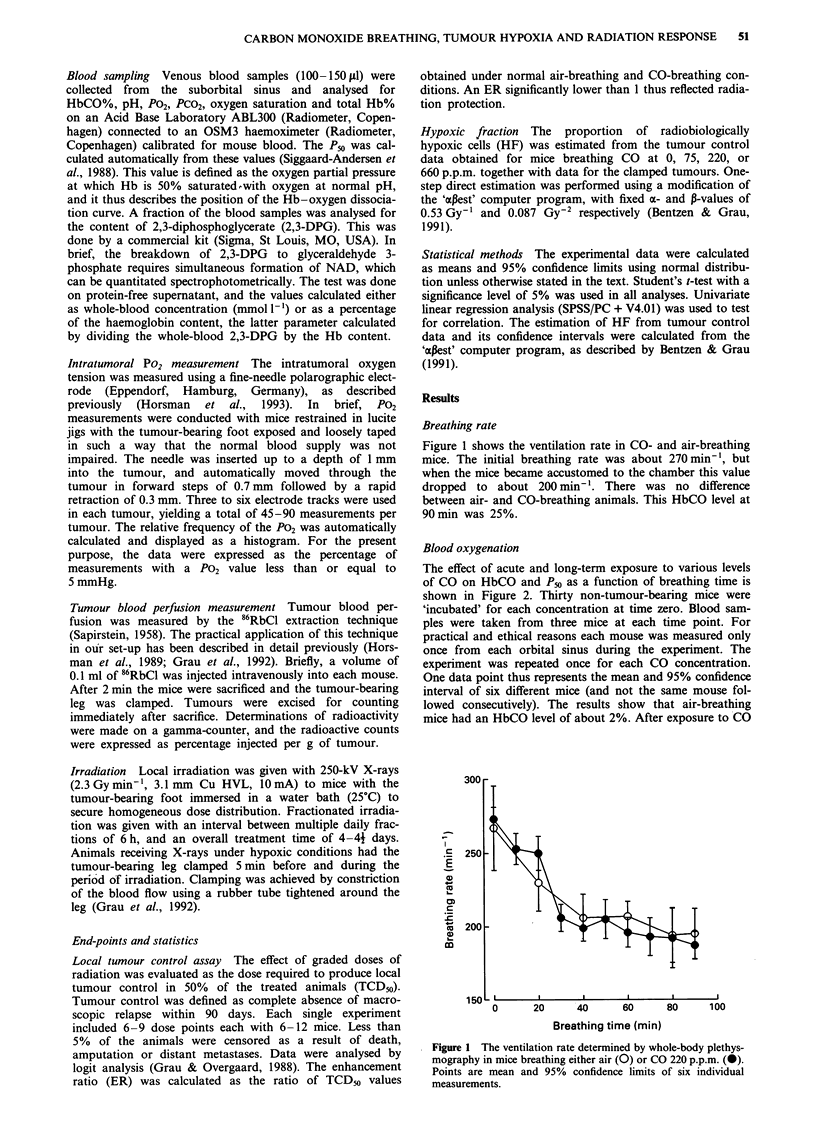
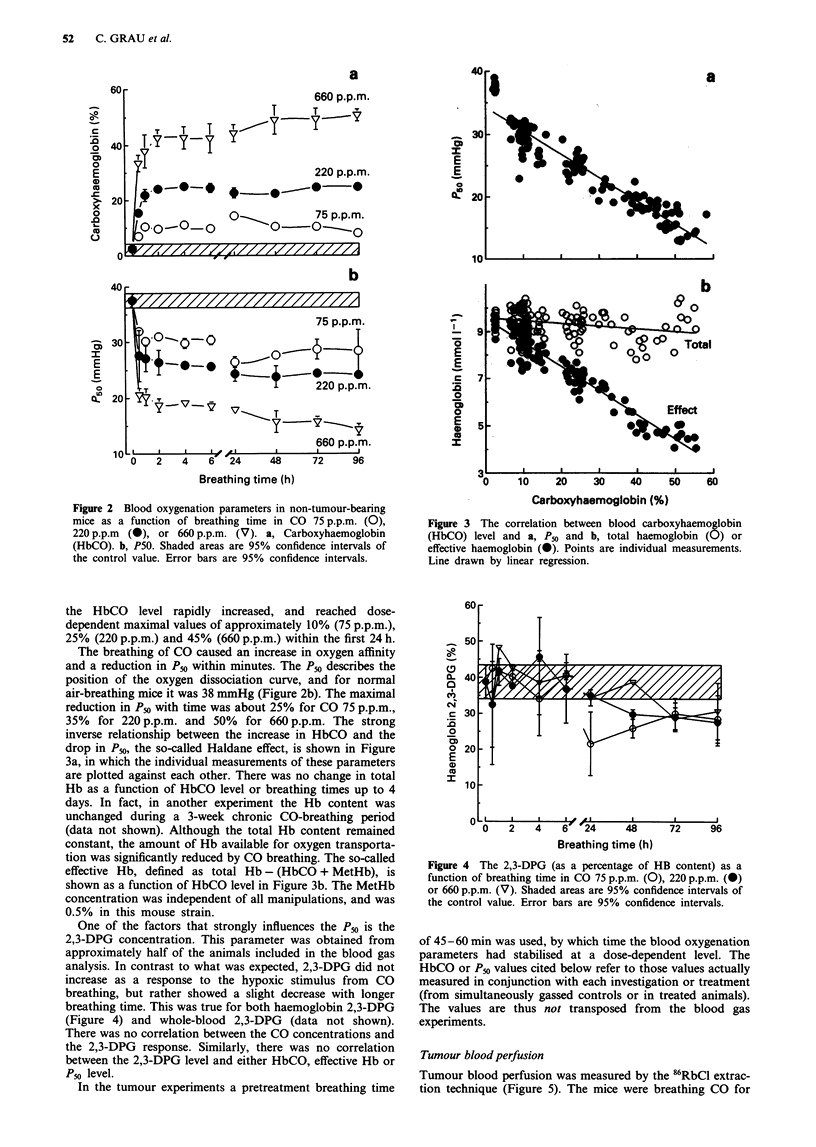
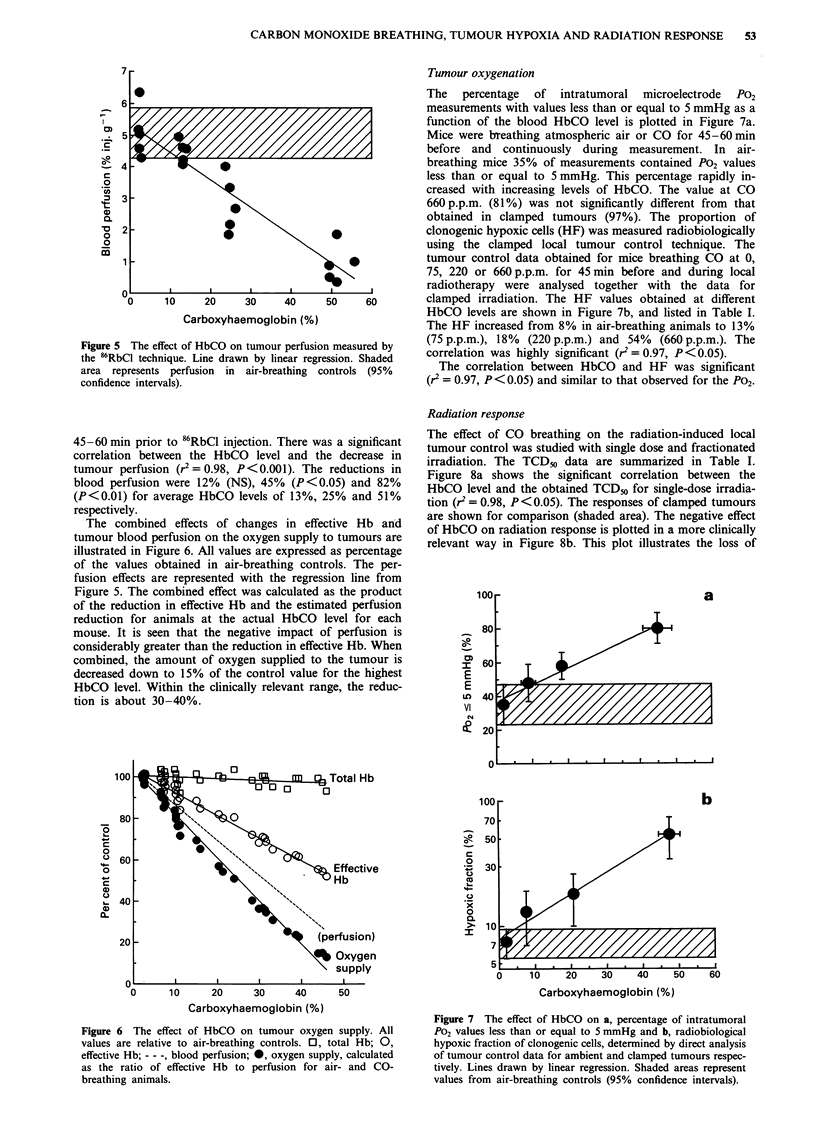
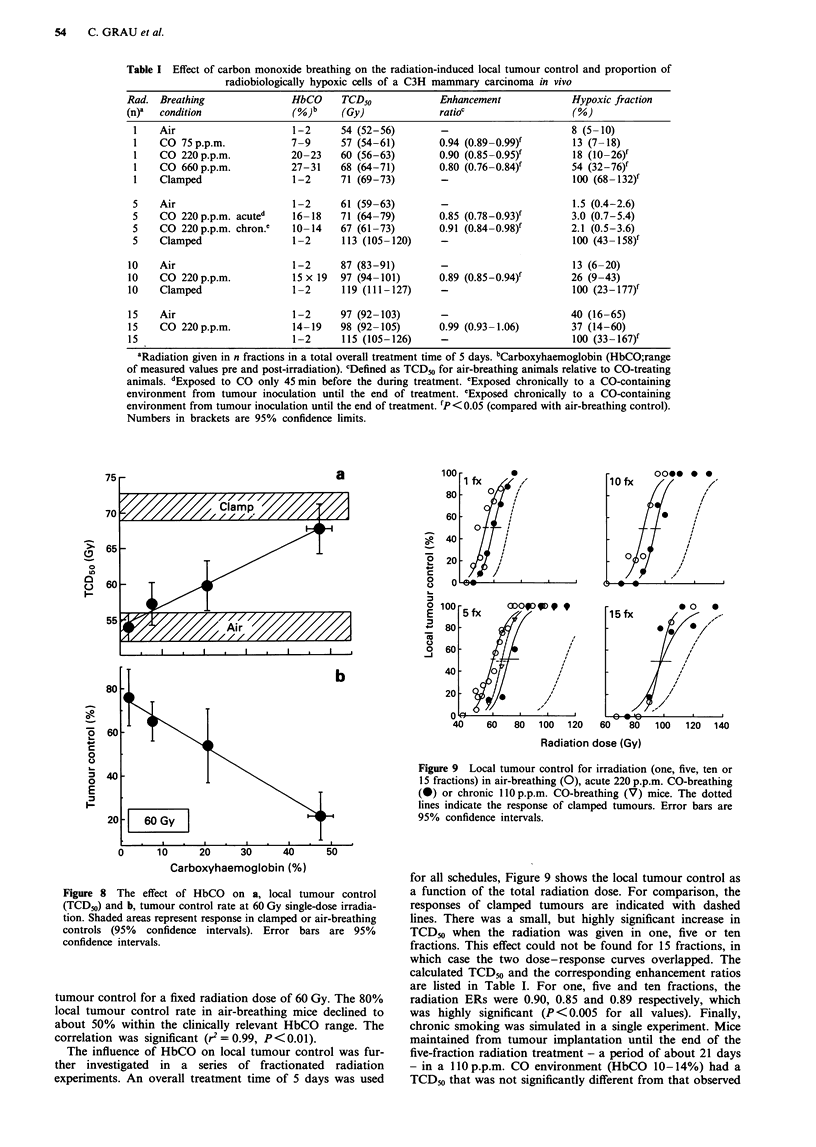
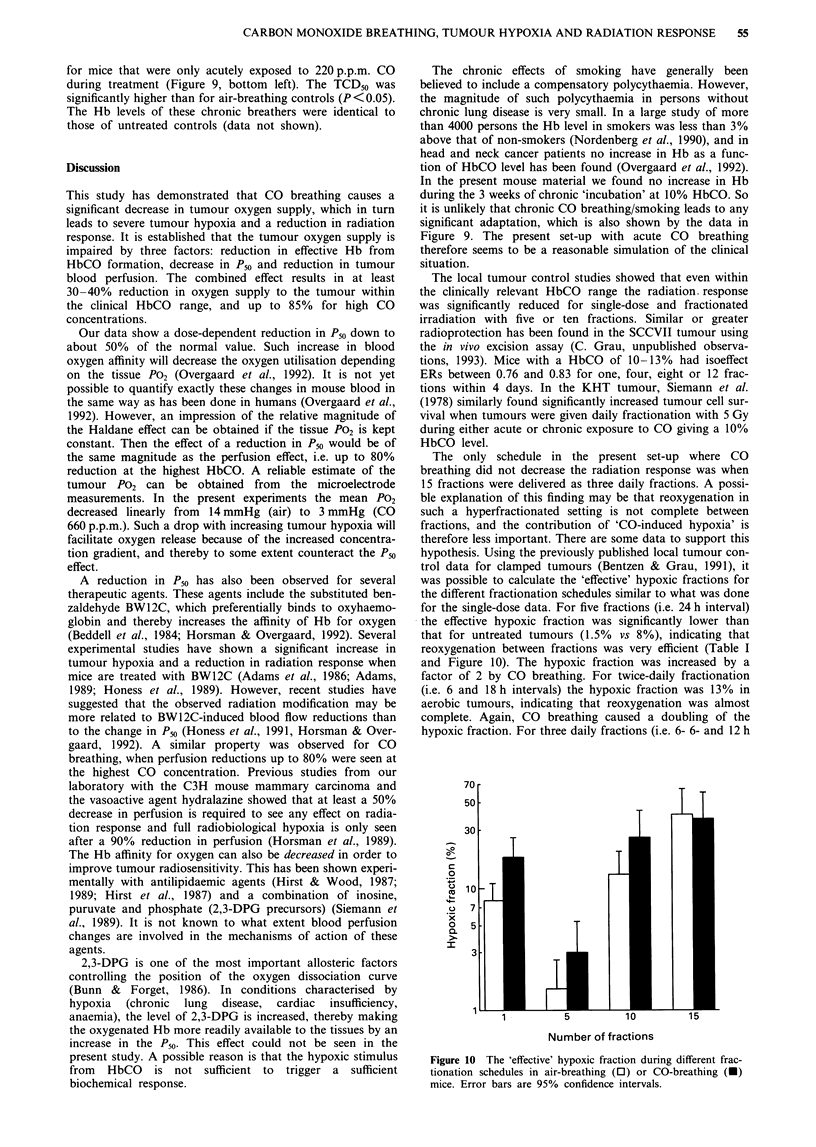
The effect of acute carbon monoxide (CO) breathing on blood oxygenation and tumour hypoxia was related to the radiation response of the C3H/Tif mammary carcinoma. Blood gas analysis showed that CO breathing caused a time- and dose-dependent formation of carboxyhaemoglobin (HbCO), a significant left shift of the oxygen dissociation curve and a reduction in tumour blood perfusion. These factors all contributed to a marked drop in tumour oxygen supply. In agreement with this, tumour hypoxia was found to be significantly increased: Microelectrode PO2 measurements showed a clear relationship between CO concentration and the proportion of low PO2 measurements (< or = 5 mmHg). The fraction of clonogenic hypoxic cells increased from 8% in air-breathing animals to 13%, 18% and 54% with 75,220 and 660 p.p.m. CO respectively. The tumour hypoxia resulted in significant radiation modification. The local tumour control after single-dose and fractionated irradiation gave TCD50 enhancement ratios (relative to air-breathing controls) of 0.90, 0.85 and 0.89 for single dose and five or ten fractions given in 5 days (P < 0.005 for all values). For 15 fractions in 5 days with 6- 6- and 12 h intervals, the TCD50 was similar in CO- and air-breathing mice, presumably as a consequence of insufficient reoxygenation during the short inter-fraction intervals. It is concluded that elevated HbCO levels to increased tumour hypoxia and that the induced hypoxia has a significant impact on the local tumour control also after fractionated irradiation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. E., Barnes D. W., du Boulay C., Loutit J. F., Cole S., Sheldon P. W., Stratford I. J., van den Aardweg G. J., Hopewell J. W., White R. D. Induction of hypoxia in normal and malignant tissues by changing the oxygen affinity of hemoglobin--implications for therapy. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1299–1302. doi: 10.1016/0360-3016(86)90158-6. [DOI] [PubMed] [Google Scholar]
- Adams G. E., Stratford I. J., Nethersell A. B., White R. D. Induction of severe tumor hypoxia by modifiers of the oxygen affinity of hemoglobin. Int J Radiat Oncol Biol Phys. 1989 May;16(5):1179–1182. doi: 10.1016/0360-3016(89)90278-2. [DOI] [PubMed] [Google Scholar]
- Beddell C. R., Goodford P. J., Kneen G., White R. D., Wilkinson S., Wootton R. Substituted benzaldehydes designed to increase the oxygen affinity of human haemoglobin and inhibit the sickling of sickle erythrocytes. Br J Pharmacol. 1984 Jun;82(2):397–407. doi: 10.1111/j.1476-5381.1984.tb10775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzen S. M., Grau C. Direct estimation of the fraction of hypoxic cells from tumour--control data obtained under aerobic and clamped conditions. Int J Radiat Biol. 1991 Jun;59(6):1435–1440. doi: 10.1080/09553009114551281. [DOI] [PubMed] [Google Scholar]
- Browman G. P., Wong G., Hodson I., Sathya J., Russell R., McAlpine L., Skingley P., Levine M. N. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med. 1993 Jan 21;328(3):159–163. doi: 10.1056/NEJM199301213280302. [DOI] [PubMed] [Google Scholar]
- Des Rochers C., Dische S., Saunders M. I. The problem of cigarette smoking in radiotherapy for cancer in the head and neck. Clin Oncol (R Coll Radiol) 1992 Jul;4(4):214–216. doi: 10.1016/s0936-6555(05)81053-2. [DOI] [PubMed] [Google Scholar]
- Gatenby R. A., Kessler H. B., Rosenblum J. S., Coia L. R., Moldofsky P. J., Hartz W. H., Broder G. J. Oxygen distribution in squamous cell carcinoma metastases and its relationship to outcome of radiation therapy. Int J Radiat Oncol Biol Phys. 1988 May;14(5):831–838. doi: 10.1016/0360-3016(88)90002-8. [DOI] [PubMed] [Google Scholar]
- Grau C., Horsman M. R., Overgaard J. Influence of carboxyhemoglobin level on tumor growth, blood flow, and radiation response in an experimental model. Int J Radiat Oncol Biol Phys. 1992;22(3):421–424. doi: 10.1016/0360-3016(92)90845-9. [DOI] [PubMed] [Google Scholar]
- Grau C., Overgaard J. Effect of cancer chemotherapy on the hypoxic fraction of a solid tumor measured using a local tumor control assay. Radiother Oncol. 1988 Dec;13(4):301–309. doi: 10.1016/0167-8140(88)90225-3. [DOI] [PubMed] [Google Scholar]
- Hirst D. G. Oxygen delivery to tumors. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1271–1277. doi: 10.1016/0360-3016(86)90152-5. [DOI] [PubMed] [Google Scholar]
- Hirst D. G., Wood P. J. Chlorophenoxy acetic acid derivatives as hemoglobin modifiers and tumor radiosensitizers. Int J Radiat Oncol Biol Phys. 1989 May;16(5):1183–1186. doi: 10.1016/0360-3016(89)90279-4. [DOI] [PubMed] [Google Scholar]
- Hirst D. G., Wood P. J., Schwartz H. C. The modification of hemoglobin affinity for oxygen and tumor radiosensitivity by antilipidemic drugs. Radiat Res. 1987 Oct;112(1):164–172. [PubMed] [Google Scholar]
- Hirst D. G., Wood P. J. The influence of haemoglobin affinity for oxygen on tumour radiosensitivity. Br J Cancer. 1987 May;55(5):487–491. doi: 10.1038/bjc.1987.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess D. J., Hu D. E., Bleehen N. M. BW12C: effects on tumour hypoxia, tumour thermosensitivity and relative tumour and normal tissue perfusion in C3H mice. Br J Cancer. 1991 Oct;64(4):715–722. doi: 10.1038/bjc.1991.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess D. J., White R. D., Nethersell A. B., Bleehen N. M. Effect of the manipulation of oxyhemoglobin status by BW12C on tumor thermosensitivity and on blood flow in tumor and normal tissues in mice. Int J Radiat Oncol Biol Phys. 1989 May;16(5):1187–1190. doi: 10.1016/0360-3016(89)90280-0. [DOI] [PubMed] [Google Scholar]
- Horsman M. R., Christensen K. L., Overgaard J. Hydralazine-induced enhancement of hyperthermic damage in a C3H mammary carcinoma in vivo. Int J Hyperthermia. 1989 Mar-Apr;5(2):123–136. doi: 10.3109/02656738909140442. [DOI] [PubMed] [Google Scholar]
- Horsman M. R., Khalil A. A., Nordsmark M., Grau C., Overgaard J. Relationship between radiobiological hypoxia and direct estimates of tumour oxygenation in a mouse tumour model. Radiother Oncol. 1993 Jul;28(1):69–71. doi: 10.1016/0167-8140(93)90188-e. [DOI] [PubMed] [Google Scholar]
- Horsman M. R., Overgaard J. BW12C-induced changes in haemoglobin-oxygen affinity in mice and its influence on the radiation response of a C3H mouse mammary carcinoma. Radiother Oncol. 1992 Sep;25(1):43–48. doi: 10.1016/0167-8140(92)90194-y. [DOI] [PubMed] [Google Scholar]
- Kucera H., Enzelsberger H., Eppel W., Weghaupt K. The influence of nicotine abuse and diabetes mellitus on the results of primary irradiation in the treatment of carcinoma of the cervix. Cancer. 1987 Jul 1;60(1):1–4. doi: 10.1002/1097-0142(19870701)60:1<1::aid-cncr2820600102>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Moulder J. E., Rockwell S. Hypoxic fractions of solid tumors: experimental techniques, methods of analysis, and a survey of existing data. Int J Radiat Oncol Biol Phys. 1984 May;10(5):695–712. doi: 10.1016/0360-3016(84)90301-8. [DOI] [PubMed] [Google Scholar]
- Nordenberg D., Yip R., Binkin N. J. The effect of cigarette smoking on hemoglobin levels and anemia screening. JAMA. 1990 Sep 26;264(12):1556–1559. [PubMed] [Google Scholar]
- Okunieff P., Hoeckel M., Dunphy E. P., Schlenger K., Knoop C., Vaupel P. Oxygen tension distributions are sufficient to explain the local response of human breast tumors treated with radiation alone. Int J Radiat Oncol Biol Phys. 1993 Jul 15;26(4):631–636. doi: 10.1016/0360-3016(93)90280-9. [DOI] [PubMed] [Google Scholar]
- Overgaard J. Effect of misonidazole and hyperthermia on the radiosensitivity of a C3H mouse mammary carcinoma and its surrounding normal tissue. Br J Cancer. 1980 Jan;41(1):10–21. doi: 10.1038/bjc.1980.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard J., Nielsen J. E., Grau C. Effect of carboxyhemoglobin on tumor oxygen unloading capacity in patients with squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1992;22(3):407–410. doi: 10.1016/0360-3016(92)90842-6. [DOI] [PubMed] [Google Scholar]
- Overgaard J. Sensitization of hypoxic tumour cells--clinical experience. Int J Radiat Biol. 1989 Nov;56(5):801–811. doi: 10.1080/09553008914552081. [DOI] [PubMed] [Google Scholar]
- SAPIRSTEIN L. A. Regional blood flow by fractional distribution of indicators. Am J Physiol. 1958 Apr;193(1):161–168. doi: 10.1152/ajplegacy.1958.193.1.161. [DOI] [PubMed] [Google Scholar]
- Siemann D. W., Alliet K. L., Macler L. M. Manipulations in the oxygen transport capacity of blood as a means of sensitizing tumors to radiation therapy. Int J Radiat Oncol Biol Phys. 1989 May;16(5):1169–1172. doi: 10.1016/0360-3016(89)90276-9. [DOI] [PubMed] [Google Scholar]
- Siemann D. W., Hill R. P., Bush R. S. Smoking: the influence of carboxyhemoglobin (HbCO) on tumor oxygenation and response to radiation. Int J Radiat Oncol Biol Phys. 1978 Jul-Aug;4(7-8):657–662. doi: 10.1016/0360-3016(78)90189-x. [DOI] [PubMed] [Google Scholar]
- Solberger O., Sorbe B. Fever, haemoglobin and smoking as prognostic factors during the treatment of cervical carcinoma by radiotherapy. Eur J Gynaecol Oncol. 1990;11(2):97–102. [PubMed] [Google Scholar]
- Testa I., Rabini R. A., Danieli G., Tranquilli A. L., Cester N., Romanini C., Bertoli E., Mazzanti L. Abnormal membrane cation transport in pregnancy-induced hypertension. Scand J Clin Lab Invest. 1988 Feb;48(1):7–13. doi: 10.1080/00365518809168289. [DOI] [PubMed] [Google Scholar]
- Vaupel P., Schlenger K., Knoop C., Höckel M. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991 Jun 15;51(12):3316–3322. [PubMed] [Google Scholar]
- van Neck J. W., Medina J. J., Onnekink C., Schwartz S. M., Bloemers H. P. Expression of basic fibroblast growth factor and fibroblast growth factor receptor genes in cultured rat aortic smooth muscle cells. Biochim Biophys Acta. 1995 Apr 4;1261(2):210–214. doi: 10.1016/0167-4781(94)00247-z. [DOI] [PubMed] [Google Scholar]
- von der Maase H., Overgaard J., Vaeth M. Effect of cancer chemotherapeutic drugs on radiation-induced lung damage in mice. Radiother Oncol. 1986 Mar;5(3):245–257. doi: 10.1016/s0167-8140(86)80054-8. [DOI] [PubMed] [Google Scholar]


