Abstract
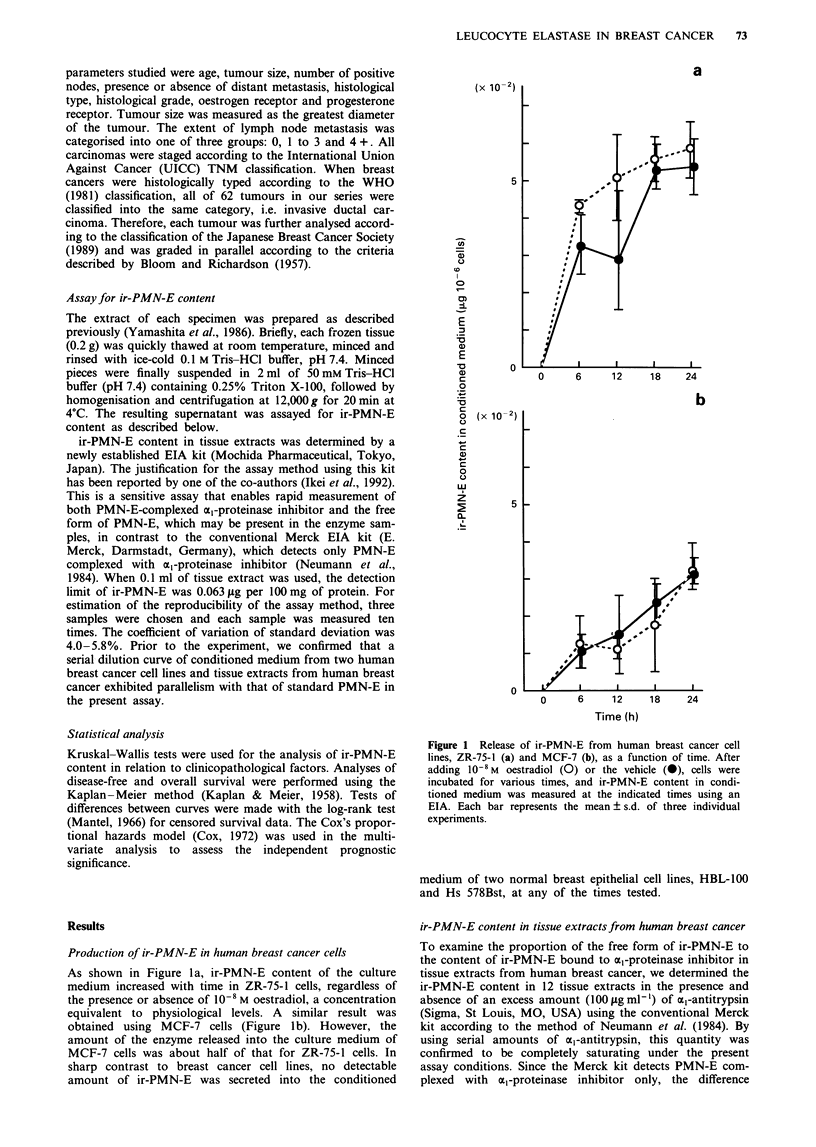
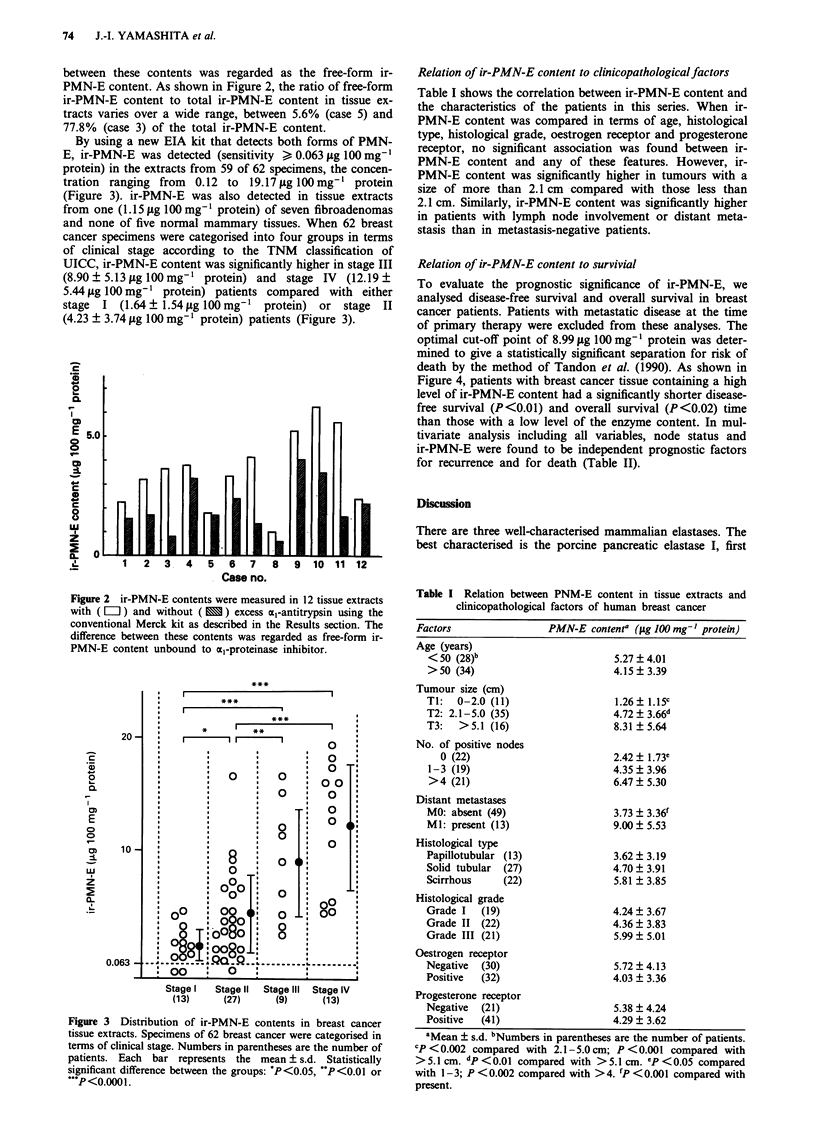
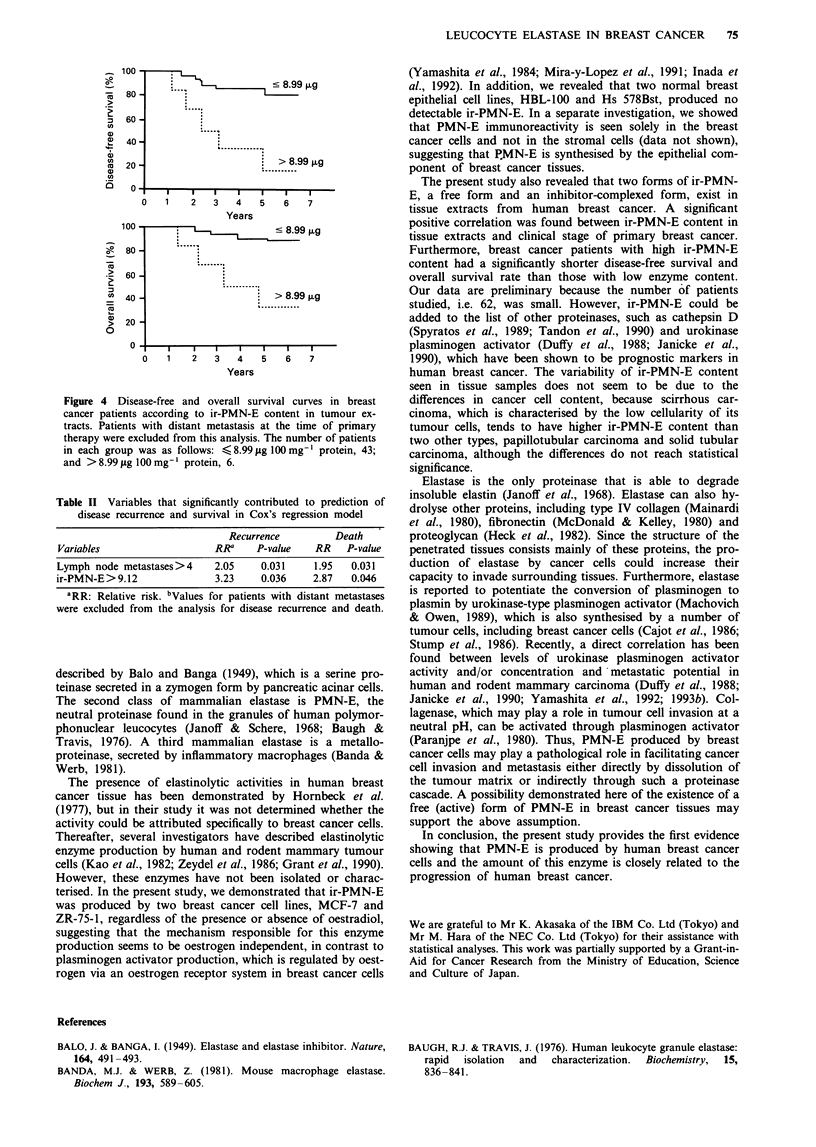
Breast cancer cells are known to express various proteolytic enzymes, which make them invasive and favour their dissemination to distant sites. However, it is unclear whether breast cancer cells have the ability to produce polymorphonuclear leucocyte elastase (PMN-E). We measured immunoreactive (ir) PMN-E content in the conditioned medium of two breast cancer cell lines, MCF-7 and ZR-75-1, and two normal breast epithelial cell lines, HBL-100 and Hs 578Bst, using a highly specific and sensitive enzyme immunoassay. Furthermore, ir-PMN-E content was determined in tissue extracts from 62 human breast cancers. ir-PMN-E content in the culture medium of MCF-7 cells and ZR-75-1 cells increased as a function of time, regardless of the presence or absence of oestradiol. On the other hand, no detectable ir-PMN-E was secreted into the culture medium of HBL-100 and Hs 578Bst cells. ir-PMN-E was detectable in 59 of 62 tissue extracts prepared from human breast cancers, the concentration ranging from 0.12 to 19.17 micrograms per 100 mg of protein. When 62 breast cancer specimens were categorised into four groups in terms of clinical stage, ir-PMN-E content in breast cancer tissue was significantly higher in stage III (8.90 +/- 5.13 micrograms 100 mg-1 protein) and stage IV (12.19 +/- 5.44 micrograms 100 mg-1 protein) patients than in stage I (1.64 +/- 1.54 micrograms 100 mg-1 protein) and stage II (4.23 +/- 3.74 micrograms 100 mg-1 protein) patients. Breast cancer patients with high levels of ir-PMN-E showed significantly shorter disease-free survival and overall survival than those with low levels of ir-PMN-E at the cut-off point of 8.99 micrograms 100 mg-1 protein. In the multivariate analysis, ir-PMN-E content was found to be a significant prognostic factor for disease recurrence and death in human breast cancer.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOOM H. J., RICHARDSON W. W. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957 Sep;11(3):359–377. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banda M. J., Werb Z. Mouse macrophage elastase. Purification and characterization as a metalloproteinase. Biochem J. 1981 Feb 1;193(2):589–605. doi: 10.1042/bj1930589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh R. J., Travis J. Human leukocyte granule elastase: rapid isolation and characterization. Biochemistry. 1976 Feb 24;15(4):836–841. doi: 10.1021/bi00649a017. [DOI] [PubMed] [Google Scholar]
- Cajot J. F., Kruithof E. K., Schleuning W. D., Sordat B., Bachmann F. Plasminogen activators, plasminogen activator inhibitors and procoagulant analyzed in twenty human tumor cell lines. Int J Cancer. 1986 Nov 15;38(5):719–727. doi: 10.1002/ijc.2910380516. [DOI] [PubMed] [Google Scholar]
- Duffy M. J. Do proteases play a role in cancer invasion and metastasis? Eur J Cancer Clin Oncol. 1987 May;23(5):583–589. doi: 10.1016/0277-5379(87)90326-9. [DOI] [PubMed] [Google Scholar]
- Duffy M. J., O'Grady P., Devaney D., O'Siorain L., Fennelly J. J., Lijnen H. J. Urokinase-plasminogen activator, a marker for aggressive breast carcinomas. Preliminary report. Cancer. 1988 Aug 1;62(3):531–533. doi: 10.1002/1097-0142(19880801)62:3<531::aid-cncr2820620315>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Grant A. J., Lerro K. A., Wu C. W. Cell associated elastase activities of rat mammary tumour cells. Biochem Int. 1990 Dec;22(6):1077–1084. [PubMed] [Google Scholar]
- Hornebeck W., Derouette J. C., Brechemier D., Adnet J. J., Robert L. Elastogenesis and elastinolytic activity in human breast cancer. Biomedicine. 1977 Feb;26(1):48–52. [PubMed] [Google Scholar]
- Inada K., Yamashita J., Matsuo S., Nakashima Y., Yamashita S., Ogawa M. Hormone control of total plasminogen activator activity is specific to malignant DMBA-induced rat mammary tumours. Br J Cancer. 1992 Apr;65(4):578–582. doi: 10.1038/bjc.1992.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff A., Scherer J. Mediators of inflammation in leukocyte lysosomes. IX. Elastinolytic activity in granules of human polymorphonuclear leukocytes. J Exp Med. 1968 Nov 1;128(5):1137–1155. doi: 10.1084/jem.128.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao R. T., Wong M., Stern R. Elastin degradation by proteases from cultured human breast cancer cells. Biochem Biophys Res Commun. 1982 Mar 15;105(1):383–389. doi: 10.1016/s0006-291x(82)80056-9. [DOI] [PubMed] [Google Scholar]
- Machovich R., Owen W. G. An elastase-dependent pathway of plasminogen activation. Biochemistry. 1989 May 16;28(10):4517–4522. doi: 10.1021/bi00436a059. [DOI] [PubMed] [Google Scholar]
- Mainardi C. L., Dixit S. N., Kang A. H. Degradation of type IV (basement membrane) collagen by a proteinase isolated from human polymorphonuclear leukocyte granules. J Biol Chem. 1980 Jun 10;255(11):5435–5441. [PubMed] [Google Scholar]
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966 Mar;50(3):163–170. [PubMed] [Google Scholar]
- McDonald J. A., Kelley D. G. Degradation of fibronectin by human leukocyte elastase. Release of biologically active fragments. J Biol Chem. 1980 Sep 25;255(18):8848–8858. [PubMed] [Google Scholar]
- Mignatti P., Robbins E., Rifkin D. B. Tumor invasion through the human amniotic membrane: requirement for a proteinase cascade. Cell. 1986 Nov 21;47(4):487–498. doi: 10.1016/0092-8674(86)90613-6. [DOI] [PubMed] [Google Scholar]
- Mira-y-Lopez R., Osborne M. P., DePalo A. J., Ossowski L. Estradiol modulation of plasminogen activator production in organ cultures of human breast carcinomas: correlation with clinical outcome of anti-estrogen therapy. Int J Cancer. 1991 Apr 1;47(6):827–832. doi: 10.1002/ijc.2910470606. [DOI] [PubMed] [Google Scholar]
- Neumann S., Hennrich N., Gunzer G., Lang H. Enzyme-linked immunoassay for human granulocyte elastase in complex with alpha 1-proteinase inhibitor. Adv Exp Med Biol. 1984;167:379–390. doi: 10.1007/978-1-4615-9355-3_33. [DOI] [PubMed] [Google Scholar]
- Paranjpe M., Engel L., Young N., Liotta L. A. Activation of human breast carcinoma collagenase through plasminogen activator. Life Sci. 1980 Apr 14;26(15):1223–1231. doi: 10.1016/0024-3205(80)90067-3. [DOI] [PubMed] [Google Scholar]
- Persky B., Ostrowski L. E., Pagast P., Ahsan A., Schultz R. M. Inhibition of proteolytic enzymes in the in vitro amnion model for basement membrane invasion. Cancer Res. 1986 Aug;46(8):4129–4134. [PubMed] [Google Scholar]
- Recklies A. D., Tiltman K. J., Stoker T. A., Poole A. R. Secretion of proteinases from malignant and nonmalignant human breast tissue. Cancer Res. 1980 Mar;40(3):550–556. [PubMed] [Google Scholar]
- Reich R., Thompson E. W., Iwamoto Y., Martin G. R., Deason J. R., Fuller G. C., Miskin R. Effects of inhibitors of plasminogen activator, serine proteinases, and collagenase IV on the invasion of basement membranes by metastatic cells. Cancer Res. 1988 Jun 15;48(12):3307–3312. [PubMed] [Google Scholar]
- Spyratos F., Maudelonde T., Brouillet J. P., Brunet M., Defrenne A., Andrieu C., Hacene K., Desplaces A., Rouëssé J., Rochefort H. Cathepsin D: an independent prognostic factor for metastasis of breast cancer. Lancet. 1989 Nov 11;2(8672):1115–1118. doi: 10.1016/s0140-6736(89)91487-6. [DOI] [PubMed] [Google Scholar]
- Stump D. C., Lijnen H. R., Collen D. Purification and characterization of single-chain urokinase-type plasminogen activator from human cell cultures. J Biol Chem. 1986 Jan 25;261(3):1274–1278. [PubMed] [Google Scholar]
- Tandon A. K., Clark G. M., Chamness G. C., Chirgwin J. M., McGuire W. L. Cathepsin D and prognosis in breast cancer. N Engl J Med. 1990 Feb 1;322(5):297–302. doi: 10.1056/NEJM199002013220504. [DOI] [PubMed] [Google Scholar]
- Turpeenniemi-Hujanen T., Thorgeirsson U. P., Hart I. R., Grant S. S., Liotta L. A. Expression of collagenase IV (basement membrane collagenase) activity in murine tumor cell hybrids that differ in metastatic potential. J Natl Cancer Inst. 1985 Jul;75(1):99–103. [PubMed] [Google Scholar]
- Yamashita J., Horiuchi S., Kimura M., Nishimura R., Akagi M. Plasminogen activator as a functional marker for estrogen dependence in human breast cancer cells. Jpn J Cancer Res. 1986 Feb;77(2):177–181. [PubMed] [Google Scholar]
- Yamashita J., Horiuchi S., Shigaki N., Fujino N., Akagi M. Estrogen-dependent plasminogen activator in 7,12-dimethylbenz[a]anthracene-induced rat mammary tumors in vivo and in vitro. Gan. 1984 Aug;75(8):681–689. [PubMed] [Google Scholar]
- Yamashita J., Inada K., Yamashita S., Matsuo S., Nakashima Y., Ogawa M. Demonstration of a possible link between high grade malignancy in dimethylbenz[a]anthracene-induced rat mammary carcinoma and increased urokinase plasminogen activator content. Int J Clin Lab Res. 1992;22(3):165–168. doi: 10.1007/BF02591417. [DOI] [PubMed] [Google Scholar]
- Yamashita J., Ogawa M., Inada K., Yamashita S., Nakashima Y., Saishoji T., Nomura K. Breast cancer prognosis is poor when total plasminogen activator activity is low. Br J Cancer. 1993 Feb;67(2):374–378. doi: 10.1038/bjc.1993.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeydel M., Nakagawa S., Biempica L., Takahashi S. Collagenase and elastase production by mouse mammary adenocarcinoma primary cultures and cloned cells. Cancer Res. 1986 Dec;46(12 Pt 1):6438–6445. [PubMed] [Google Scholar]


