Abstract
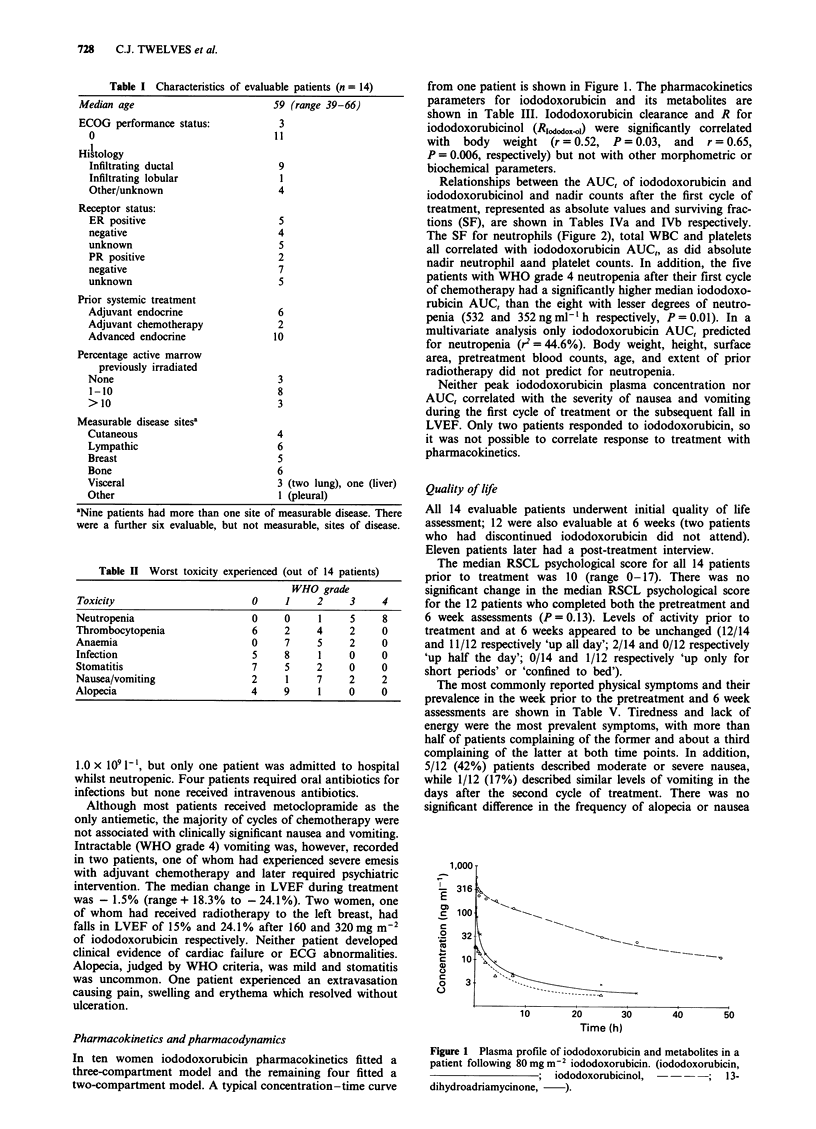
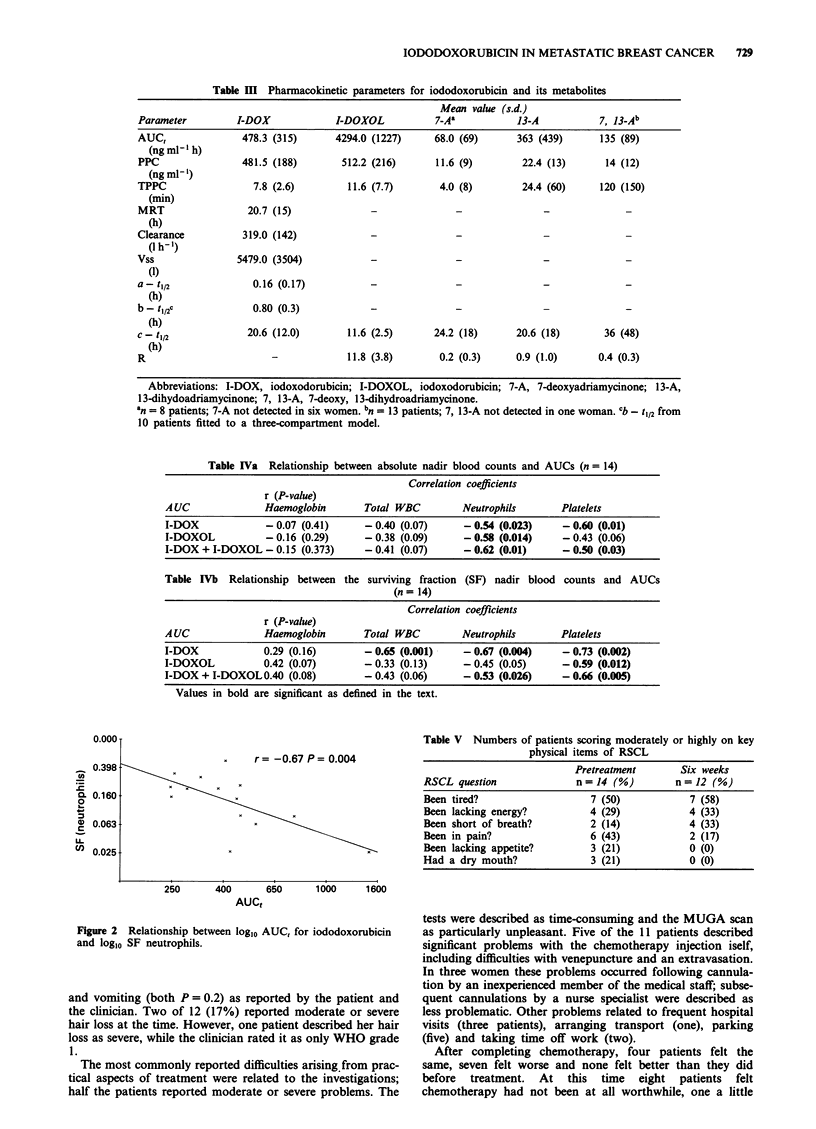
Iododoxorubicin 80 mg m-2 i.v. was given 3 weekly for a maximum of six cycles as first-line chemotherapy to 14 evaluable women with metastatic breast cancer. The response rate was 14% (95% confidence intervals 4-40%); median time to progression was 3.5 months (range 0.7 to > 9.3) and median survival was 10.2 months (range 2.3 to > 20.4). Neutropenia was the main toxicity but was not associated with severe sepsis. Two patients had a significant (> 10%) but asymptomatic fall in cardiac ejection fraction; other toxicities were mild. Plasma pharmacokinetics was studied during the first cycle of treatment. Iododoxorubicin was extensively metabolised to iododoxorubicinol. Neutropenia and thrombocytopenia were both significantly correlated with the area under the concentration-time curve (AUC) for iododoxorubicin and the total AUC for iododoxorubicin and iododoxorubicinol. Quality of life (QOL), evaluated by self-report questionnaire and interview, showed little evidence of benefit in terms of physical symptom relief, level of activity, psychological symptoms or global evaluation of QOL during treatment. Iododoxorubicin is subjectively less toxic than standard anthracyclines, but at the dose and schedule used has limited activity in metastatic breast cancer, possibly because iododoxorubicinol is not clinically active.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abernethy D. R., Divoll M., Greenblatt D. J., Ameer B. Obesity, sex, and acetaminophen disposition. Clin Pharmacol Ther. 1982 Jun;31(6):783–790. doi: 10.1038/clpt.1982.111. [DOI] [PubMed] [Google Scholar]
- Ackland S. P., Ratain M. J., Vogelzang N. J., Choi K. E., Ruane M., Sinkule J. A. Pharmacokinetics and pharmacodynamics of long-term continuous-infusion doxorubicin. Clin Pharmacol Ther. 1989 Apr;45(4):340–347. doi: 10.1038/clpt.1989.39. [DOI] [PubMed] [Google Scholar]
- Barbieri B., Giuliani F. C., Bordoni T., Casazza A. M., Geroni C., Bellini O., Suarato A., Gioia B., Penco S., Arcamone F. Chemical and biological characterization of 4'-iodo-4'-deoxydoxorubicin. Cancer Res. 1987 Aug 1;47(15):4001–4006. [PubMed] [Google Scholar]
- Benet L. Z., Galeazzi R. L. Noncompartmental determination of the steady-state volume of distribution. J Pharm Sci. 1979 Aug;68(8):1071–1074. doi: 10.1002/jps.2600680845. [DOI] [PubMed] [Google Scholar]
- Danesi R., Marchetti A., Bernardini N., La Rocca R. V., Bevilacqua G., Del Tacca M. Cardiac toxicity and antitumor activity of 4'-deoxy-4'-iodo-doxorubicinol. Cancer Chemother Pharmacol. 1990;26(6):403–408. doi: 10.1007/BF02994089. [DOI] [PubMed] [Google Scholar]
- ELLIS R. E. The distribution of active bone marrow in the adult. Phys Med Biol. 1961 Jan;5:255–258. doi: 10.1088/0031-9155/5/3/302. [DOI] [PubMed] [Google Scholar]
- Formelli F., Carsana R., Pollini C. Pharmacokinetics of 4'-deoxy-4'-iodo-doxorubicin in plasma and tissues of tumor-bearing mice compared with doxorubicin. Cancer Res. 1987 Oct 15;47(20):5401–5406. [PubMed] [Google Scholar]
- Freedman L. S., Workman P. When can the infusion period be safely ignored in the estimation of pharmacokinetic parameters of drugs in humans? Cancer Chemother Pharmacol. 1988;22(2):95–103. doi: 10.1007/BF00257304. [DOI] [PubMed] [Google Scholar]
- Gianni L., Capri G., Greco M., Villani F., Brambilla C., Luini A., Crippa F., Bonadonna G. Activity and toxicity of 4'-iodo-4'-deoxydoxorubicin in patients with advanced breast cancer. Ann Oncol. 1991 Nov-Dec;2(10):719–725. doi: 10.1093/oxfordjournals.annonc.a057849. [DOI] [PubMed] [Google Scholar]
- Gianni L., Viganò L., Surbone A., Ballinari D., Casali P., Tarella C., Collins J. M., Bonadonna G. Pharmacology and clinical toxicity of 4'-iodo-4'-deoxydoxorubicin: an example of successful application of pharmacokinetics to dose escalation in phase I trials. J Natl Cancer Inst. 1990 Mar 21;82(6):469–477. doi: 10.1093/jnci/82.6.469. [DOI] [PubMed] [Google Scholar]
- Johnston A., Woollard R. C. STRIPE: an interactive computer program for the analysis of drug pharmacokinetics. J Pharmacol Methods. 1983 May;9(3):193–199. doi: 10.1016/0160-5402(83)90038-4. [DOI] [PubMed] [Google Scholar]
- Judson I. R. Phase II studies: wrong doses, wrong patients? Eur J Cancer. 1991;27(10):1198–1200. doi: 10.1016/0277-5379(91)90080-w. [DOI] [PubMed] [Google Scholar]
- Loveless H., Arena E., Felsted R. L., Bachur N. R. Comparative mammalian metabolism of adriamycin and daunorubicin. Cancer Res. 1978 Mar;38(3):593–598. [PubMed] [Google Scholar]
- Mross K., Mayer U., Langenbuch T., Hamm K., Burk K., Hossfeld D. Toxicity, pharmacokinetics and metabolism of iododoxorubicin in cancer patients. Eur J Cancer. 1990;26(11-12):1156–1162. doi: 10.1016/0277-5379(90)90276-y. [DOI] [PubMed] [Google Scholar]
- Mross K. New anthracycline derivatives: what for? Eur J Cancer. 1991;27(12):1542–1544. doi: 10.1016/0277-5379(91)90409-7. [DOI] [PubMed] [Google Scholar]
- Robert J., Armand J. P., Huet S., Klink-Alakl M., Recondo G., Hurteloup P. Pharmacokinetics and metabolism of 4'-iodo-4'-deoxy-doxorubicin in humans. J Clin Oncol. 1992 Jul;10(7):1183–1190. doi: 10.1200/JCO.1992.10.7.1183. [DOI] [PubMed] [Google Scholar]
- Schott B., Vrignaud P., Ries C., Robert J., Londos-Gagliardi D. Cellular pharmacology of 4'-iodo-4'-deoxydoxorubicin. Br J Cancer. 1990 Apr;61(4):543–547. doi: 10.1038/bjc.1990.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartsmann G., Pinedo H. M. Clinical trials in advanced breast cancer. Eur J Cancer Clin Oncol. 1987 Jun;23(6):595–597. doi: 10.1016/0277-5379(87)90251-3. [DOI] [PubMed] [Google Scholar]
- Schwartsmann G., Pinedo H. M. Multicenter vs single center phase II evaluation of 4'-iodo-4'-deoxydoxorubicin in advanced breast cancer. Ann Oncol. 1991 Nov-Dec;2(10):703–706. doi: 10.1093/oxfordjournals.annonc.a057845. [DOI] [PubMed] [Google Scholar]
- Schwartz J. E., Salmon S. E. Comparative in vitro activity of 4'-deoxy-4'-iododoxorubicin and other anthracyclines in the human tumor clonogenic assay. Invest New Drugs. 1987;5(3):231–234. doi: 10.1007/BF00175292. [DOI] [PubMed] [Google Scholar]
- Sessa C., Calabresi F., Cavalli F., Cerny T., Liati P., Skovsgaard T., Sorio R., Kaye S. B. Phase II studies of 4'-iodo-4'-deoxydoxorubicin in advanced non-small cell lung, colon and breast cancers. Ann Oncol. 1991 Nov-Dec;2(10):727–731. doi: 10.1093/oxfordjournals.annonc.a057851. [DOI] [PubMed] [Google Scholar]
- Skipper H. E., Schabel F. M., Jr, Mellett L. B., Montgomery J. A., Wilkoff L. J., Lloyd H. H., Brockman R. W. Implications of biochemical, cytokinetic, pharmacologic, and toxicologic relationships in the design of optimal therapeutic schedules. Cancer Chemother Rep. 1970 Dec;54(6):431–450. [PubMed] [Google Scholar]
- Supino R., Mariani M., Prosperi E., Parmiani G. Lack of cross-resistance of a doxorubicin-resistant B16 melanoma line with 4'-deoxy-4'-iodo-doxorubicin. Cancer Chemother Pharmacol. 1988;21(3):251–254. doi: 10.1007/BF00262780. [DOI] [PubMed] [Google Scholar]
- de Haes J. C., van Knippenberg F. C., Neijt J. P. Measuring psychological and physical distress in cancer patients: structure and application of the Rotterdam Symptom Checklist. Br J Cancer. 1990 Dec;62(6):1034–1038. doi: 10.1038/bjc.1990.434. [DOI] [PMC free article] [PubMed] [Google Scholar]


