Abstract
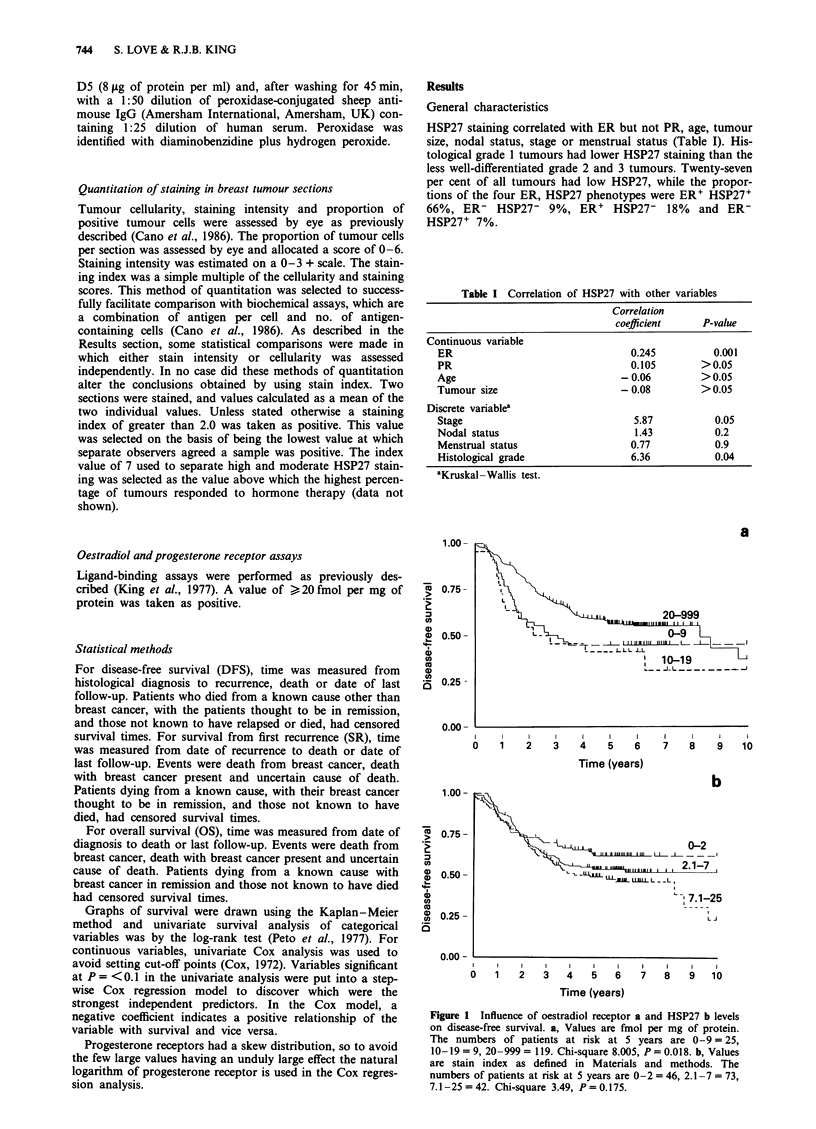
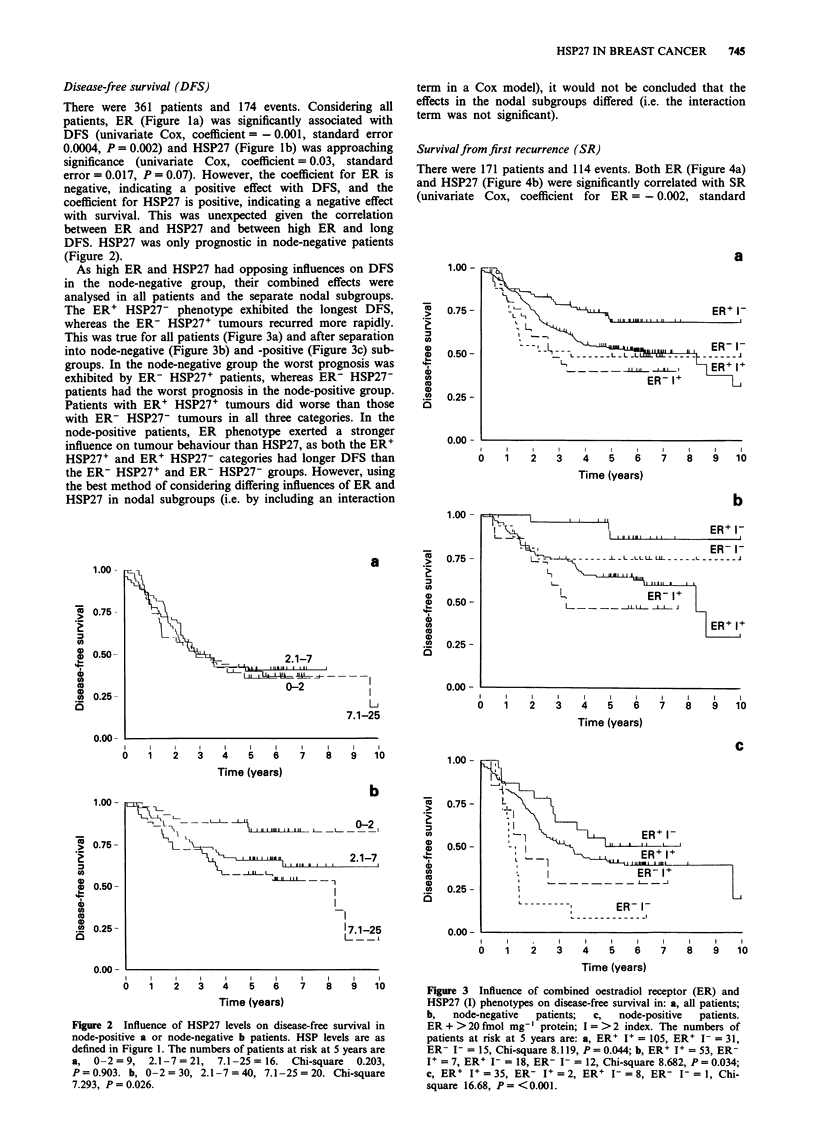
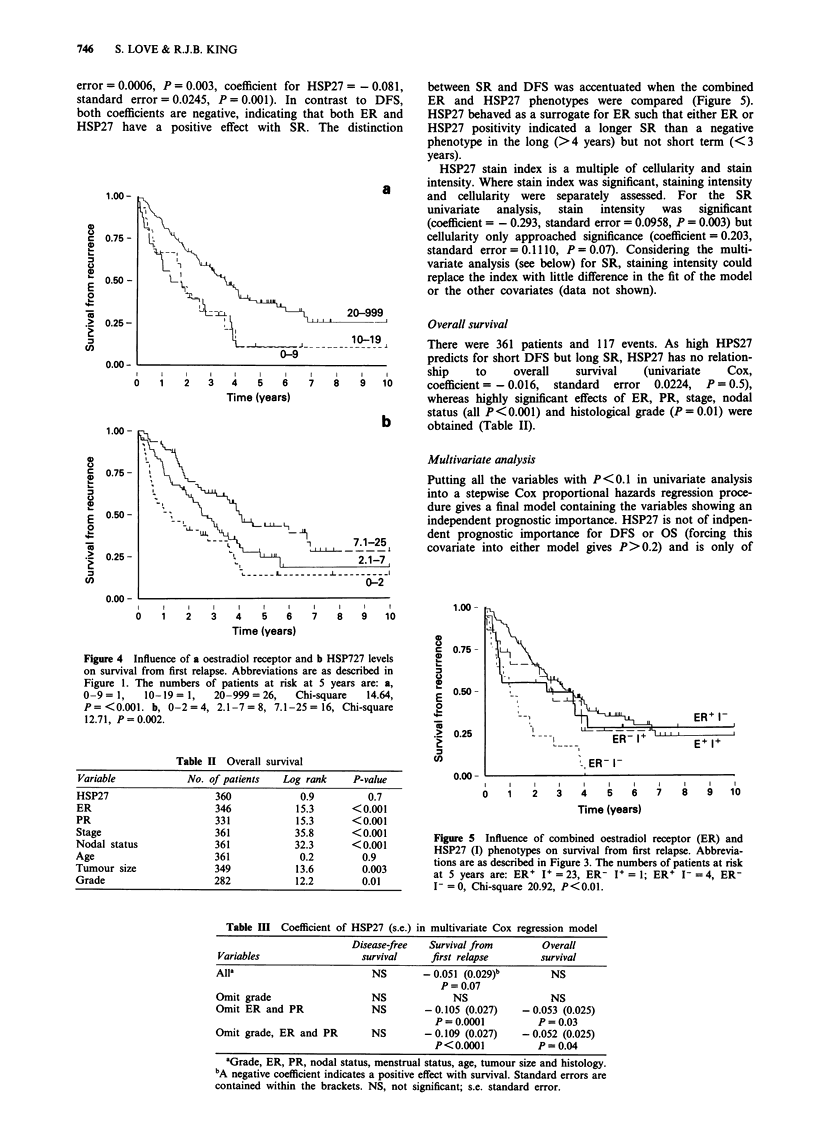
This paper describes a prospective immunohistochemical analysis of 27 kDa heat shock protein (HSP27) in 361 patients with primary breast cancer in relation to disease-free survival (DFS) and survival from first relapse (SR). Oestradiol (ER) and progesterone (PR) receptors were also quantitated and related to the HSP27 data. While ER positively predicted a good outcome for both DFS and SR, HSP27 positivity predicted a prolonged SR but short DFS. The association between HSP27 and DFS only attained statistical significance in node-negative patients. Subgroup analysis reinforced the complementary relationship of HSP27 and ER for SR and opposing influences for DFS. In both node-negative and node-positive women, ER+ HSP27- patients had a longer DFS than ER- HSP27+ counterparts. There was no relationship between HSP27 and overall survival. HSP27 staining was highly correlated with ER but not PR, patient age, tumour size or menstrual status. There was a marginal correlation (P = 0.04) with histological grade with well-differentiated tumours having the highest HSP27. Cox multivariate regression analysis of the contribution of HSP27 in the presence of data on ER, PR, stage, nodal status and histological grade indicated that HSP27 was not of independent prognostic importance for DFS or overall survival and was only of borderline significance for OS (P < 0.07). However, in the absence of ER and PR data, HSP27 staining is an effective way of getting the same prognostic information. HSP27 staining appears to correlate with different biological features in early and advanced breast, high HSP27 being linked with short DFS in node-negative patients but with prolonged survival from first recurrence.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., McGuire W. L. Quantitative enzyme-linked immunosorbent assay for the estrogen-regulated Mr 24,000 protein in human breast tumors: correlation with estrogen and progesterone receptors. Cancer Res. 1985 Jun;45(6):2445–2449. [PubMed] [Google Scholar]
- Cano A., Coffer A. I., Adatia R., Millis R. R., Rubens R. D., King R. J. Histochemical studies with an estrogen receptor-related protein in human breast tumors. Cancer Res. 1986 Dec;46(12 Pt 1):6475–6480. [PubMed] [Google Scholar]
- Ciocca D. R., Adams D. J., Edwards D. P., Bjercke R. J., McGuire W. L. Distribution of an estrogen-induced protein with a molecular weight of 24,000 in normal and malignant human tissues and cells. Cancer Res. 1983 Mar;43(3):1204–1210. [PubMed] [Google Scholar]
- Ciocca D. R., Fuqua S. A., Lock-Lim S., Toft D. O., Welch W. J., McGuire W. L. Response of human breast cancer cells to heat shock and chemotherapeutic drugs. Cancer Res. 1992 Jul 1;52(13):3648–3654. [PubMed] [Google Scholar]
- Ciocca D. R., Luque E. H. Immunological evidence for the identity between the hsp27 estrogen-regulated heat shock protein and the p29 estrogen receptor-associated protein in breast and endometrial cancer. Breast Cancer Res Treat. 1991 Dec;20(1):33–42. doi: 10.1007/BF01833355. [DOI] [PubMed] [Google Scholar]
- Coffer A. I., King R. J. Characterization of p29, an estrogen-receptor associated tumor marker. J Steroid Biochem. 1988 Nov;31(5):745–750. doi: 10.1016/0022-4731(88)90281-6. [DOI] [PubMed] [Google Scholar]
- Coffer A. I., Lewis K. M., Brockas A. J., King R. J. Monoclonal antibodies against a component related to soluble estrogen receptor. Cancer Res. 1985 Aug;45(8):3686–3693. [PubMed] [Google Scholar]
- Damstrup L., Andersen J., Kufe D. W., Hayes D. F., Poulsen H. S. Immunocytochemical determination of the estrogen-regulated proteins Mr 24,000, Mr 52,000 and DF3 breast cancer associated antigen: clinical value in advanced breast cancer and correlation with estrogen receptor. Ann Oncol. 1992 Jan;3(1):71–77. doi: 10.1093/oxfordjournals.annonc.a058078. [DOI] [PubMed] [Google Scholar]
- Davidson N. E., Abeloff M. D. Adjuvant systemic therapy in women with early-stage breast cancer at high risk for relapse. J Natl Cancer Inst. 1992 Mar 4;84(5):301–305. doi: 10.1093/jnci/84.5.301. [DOI] [PubMed] [Google Scholar]
- Dunn D. K., Whelan R. D., Hill B., King R. J. Relationship of HSP27 and oestrogen receptor in hormone sensitive and insensitive cell lines. J Steroid Biochem Mol Biol. 1993 Oct;46(4):469–479. doi: 10.1016/0960-0760(93)90101-2. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. The emergence of the chaperone machines. Trends Biochem Sci. 1992 Aug;17(8):295–299. doi: 10.1016/0968-0004(92)90439-g. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Girling A., Caleffi M., King R. J., Millis R. R. Immunohistochemical study of D5 antigen (an oestrogen receptor related protein) in normal breast, benign breast disease, and mammary carcinoma in situ. J Clin Pathol. 1988 Apr;41(4):448–453. doi: 10.1136/jcp.41.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J. D., Jones J. A., Ellis I. O., Morris D. L. Oestrogen receptor D5 antibody is an independent negative prognostic factor in gastric cancer. Br J Surg. 1991 Mar;78(3):334–336. doi: 10.1002/bjs.1800780321. [DOI] [PubMed] [Google Scholar]
- Henry R. J., Goodman J. D., Godley M., Raju K. S., Coffer A. I., King R. J. Immunohistochemical study of cytoplasmic oestradiol receptor in normal, dysplastic and malignant cervical tissue. Br J Obstet Gynaecol. 1988 Sep;95(9):927–932. doi: 10.1111/j.1471-0528.1988.tb06582.x. [DOI] [PubMed] [Google Scholar]
- Huot J., Roy G., Lambert H., Chrétien P., Landry J. Increased survival after treatments with anticancer agents of Chinese hamster cells expressing the human Mr 27,000 heat shock protein. Cancer Res. 1991 Oct 1;51(19):5245–5252. [PubMed] [Google Scholar]
- Jordan V. C., Wolf M. F., Mirecki D. M., Whitford D. A., Welshons W. V. Hormone receptor assays: clinical usefulness in the management of carcinoma of the breast. Crit Rev Clin Lab Sci. 1988;26(2):97–152. doi: 10.3109/10408368809106860. [DOI] [PubMed] [Google Scholar]
- Kato M., Herz F., Kato S., Hirano A. Expression of stress-response (heat-shock) protein 27 in human brain tumors: an immunohistochemical study. Acta Neuropathol. 1992;83(4):420–422. doi: 10.1007/BF00713535. [DOI] [PubMed] [Google Scholar]
- King R. J., Finley J. R., Coffer A. I., Millis R. R., Rubens R. D. Characterization and biological relevance of a 29-kDa, oestrogen receptor-related protein. J Steroid Biochem. 1987;27(1-3):471–475. doi: 10.1016/0022-4731(87)90342-6. [DOI] [PubMed] [Google Scholar]
- King R. J., Hayward J. L., Kumaoka S., Yamamoto H. Comparison of soluble oestrogen and progestin receptor content of primary breast tumours from Japan and Britain. Eur J Cancer. 1977 Sep;13(9):967–970. doi: 10.1016/0014-2964(77)90174-8. [DOI] [PubMed] [Google Scholar]
- King R. J. William L. McGuire Memorial Symposium. Estrogen and progestin effects in human breast carcinogenesis. Breast Cancer Res Treat. 1993;27(1-2):3–15. doi: 10.1007/BF00683189. [DOI] [PubMed] [Google Scholar]
- McGuire W. L. Adjuvant therapy of node-negative breast cancer. N Engl J Med. 1989 Feb 23;320(8):525–527. doi: 10.1056/NEJM198902233200811. [DOI] [PubMed] [Google Scholar]
- McGuire W. L. Prognostic factors for recurrence and survival in human breast cancer. Breast Cancer Res Treat. 1987 Oct;10(1):5–9. doi: 10.1007/BF01806129. [DOI] [PubMed] [Google Scholar]
- Mendelsohn M. E., Zhu Y., O'Neill S. The 29-kDa proteins phosphorylated in thrombin-activated human platelets are forms of the estrogen receptor-related 27-kDa heat shock protein. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11212–11216. doi: 10.1073/pnas.88.24.11212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson H., Baldetorp B., Borg A., Dalberg M., Fernö M., Killander D., Olsson H. Indicators of prognosis in node-negative breast cancer. N Engl J Med. 1990 Apr 12;322(15):1045–1053. doi: 10.1056/NEJM199004123221505. [DOI] [PubMed] [Google Scholar]
- Strahler J. R., Kuick R., Hanash S. M. Diminished phosphorylation of a heat shock protein (HSP 27) in infant acute lymphoblastic leukemia. Biochem Biophys Res Commun. 1991 Feb 28;175(1):134–142. doi: 10.1016/s0006-291x(05)81211-2. [DOI] [PubMed] [Google Scholar]
- Sunderland M. C., McGuire W. L. Oncogenes as clinical prognostic indicators. Cancer Treat Res. 1991;53:3–22. doi: 10.1007/978-1-4615-3940-7_1. [DOI] [PubMed] [Google Scholar]
- Thor A., Benz C., Moore D., 2nd, Goldman E., Edgerton S., Landry J., Schwartz L., Mayall B., Hickey E., Weber L. A. Stress response protein (srp-27) determination in primary human breast carcinomas: clinical, histologic, and prognostic correlations. J Natl Cancer Inst. 1991 Feb 6;83(3):170–178. doi: 10.1093/jnci/83.3.170. [DOI] [PubMed] [Google Scholar]
- Whelan R. D., Hill B. T. Differential expression of steroid receptors, hsp27, and pS2 in a series of drug resistant human breast tumor cell lines derived following exposure to antitumor drugs or to fractionated X-irradiation. Breast Cancer Res Treat. 1993;26(1):23–39. doi: 10.1007/BF00682697. [DOI] [PubMed] [Google Scholar]
- Zantema A., de Jong E., Lardenoije R., van der Eb A. J. The expression of heat shock protein hsp27 and a complexed 22-kilodalton protein is inversely correlated with oncogenicity of adenovirus-transformed cells. J Virol. 1989 Aug;63(8):3368–3375. doi: 10.1128/jvi.63.8.3368-3375.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


