Abstract
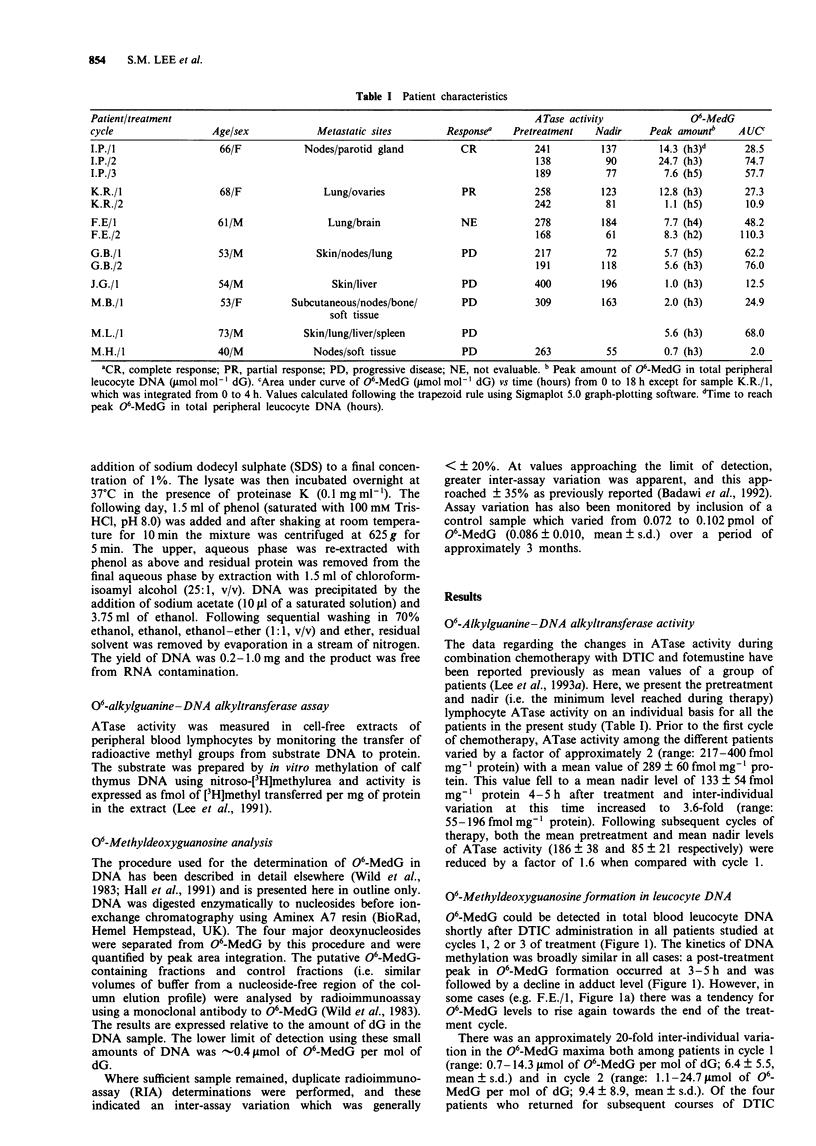
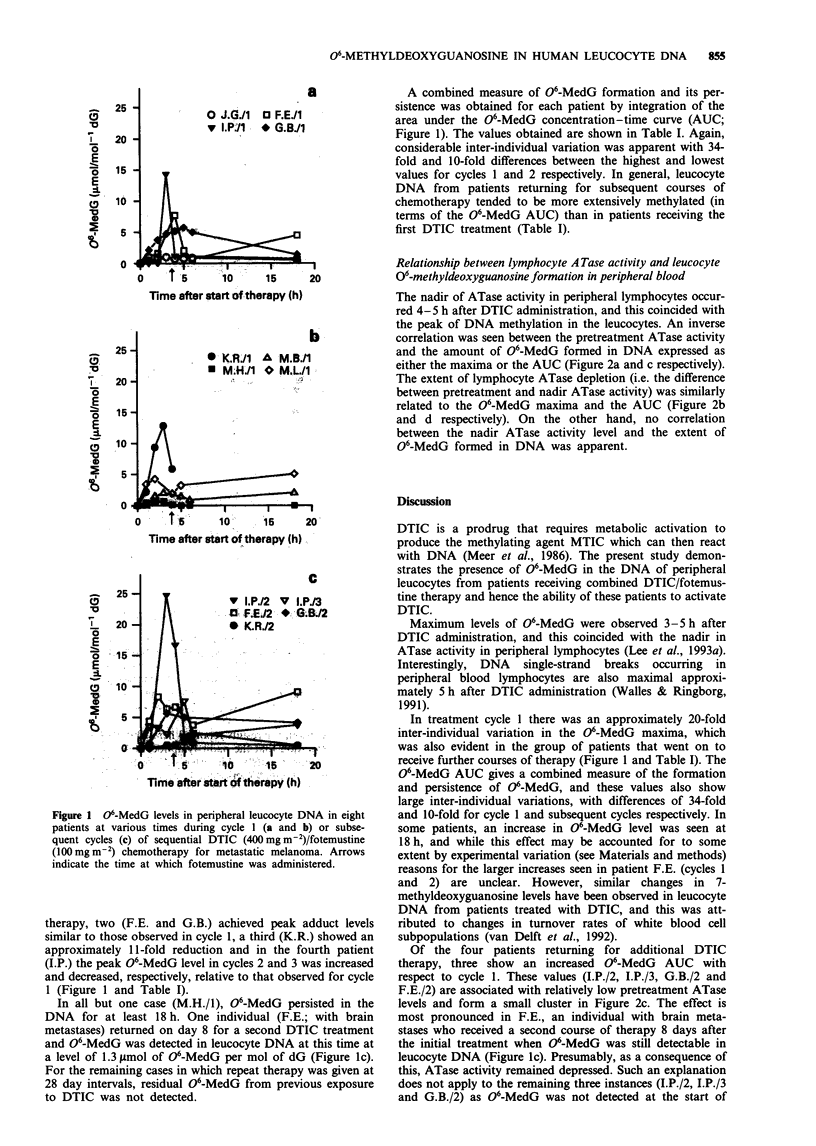
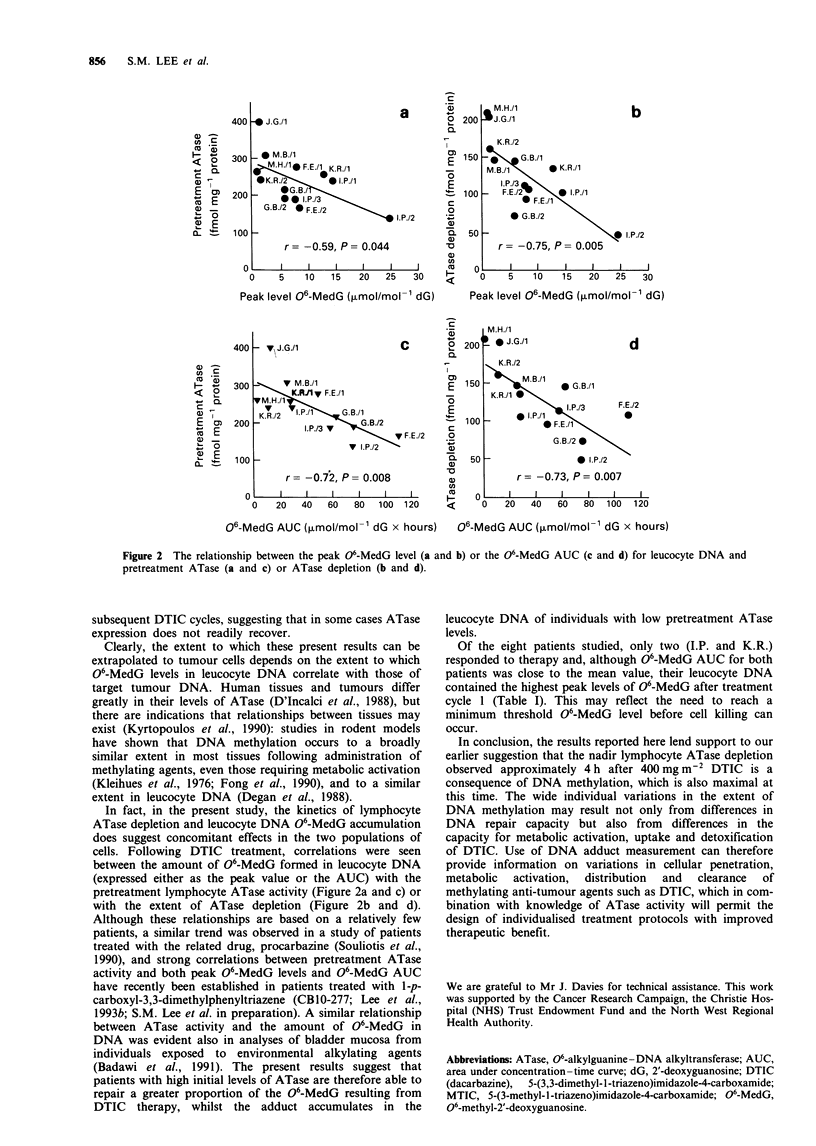
There is increasing evidence to indicate that O6-methyldeoxyguanosine (O6-MedG) formation in DNA is a critical cytotoxic event following exposure to certain anti-tumour alkylating agents and that the DNA repair protein O6-alkylguanine-DNA alkyltransferase (ATase) can confer resistance to these agents. We recently demonstrated a wide inter-individual variation in the depletion and subsequent regeneration of ATase in human peripheral blood lymphocytes following sequential DTIC (400 mg m-2) and fotemustine (100 mg m-2) treatment, with the nadir ATase activity occurring approximately 4 h after DTIC administration. We have now measured the formation and loss of O6-methyldeoxyguanosine (O6-MedG) in the DNA of peripheral leucocytes of eight patients receiving this treatment regimen. O6-MedG could be detected within 1 h and maximal levels occurred approximately 3-5 h after DTIC administration. Following the first treatment cycle, considerable inter-individual variation was observed in the peak O6-MedG levels, with values ranging from 0.71 to 14.3 mumol of O6-MedG per mol of dG (6.41 +/- 5.53, mean +/- s.d.). Inter- and intra-individual variation in the extent of O6-MedG formation was also seen in patients receiving additional treatment cycles. This may be a consequence of inter-patient differences in the capacity for metabolism of DTIC to release a methylating intermediate and could be one of the determinants of clinical response. Both the pretreatment ATase levels and the extent of ATase depletion were inversely correlated with the amount of O6-MedG formed in leucocyte DNA when expressed either as peak levels (r = -0.59 and -0.75 respectively) or as the area under the concentration-time curve (r = -0.72 and -0.73 respectively). One complete and one partial clinical response were seen, and these occurred in the two patients with the highest O6-MedG levels in the peripheral leucocyte DNA, although the true significance of this observation has yet to be established.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badawi A. F., Mostafa M. H., Aboul-Azm T., Haboubi N. Y., O'Connor P. J., Cooper D. P. Promutagenic methylation damage in bladder DNA from patients with bladder cancer associated with schistosomiasis and from normal individuals. Carcinogenesis. 1992 May;13(5):877–881. doi: 10.1093/carcin/13.5.877. [DOI] [PubMed] [Google Scholar]
- Brennand J., Margison G. P. Expression in mammalian cells of a truncated Escherichia coli gene coding for O6-alkylguanine alkyltransferase reduces the toxic effects of alkylating agents. Carcinogenesis. 1986 Dec;7(12):2081–2084. doi: 10.1093/carcin/7.12.2081. [DOI] [PubMed] [Google Scholar]
- Catapano C. V., Broggini M., Erba E., Ponti M., Mariani L., Citti L., D'Incalci M. In vitro and in vivo methazolastone-induced DNA damage and repair in L-1210 leukemia sensitive and resistant to chloroethylnitrosoureas. Cancer Res. 1987 Sep 15;47(18):4884–4889. [PubMed] [Google Scholar]
- Comis R. L. DTIC (NSC-45388) in malignant melanoma: a perspective. Cancer Treat Rep. 1976 Feb;60(2):165–176. [PubMed] [Google Scholar]
- D'Incalci M., Citti L., Taverna P., Catapano C. V. Importance of the DNA repair enzyme O6-alkyl guanine alkyltransferase (AT) in cancer chemotherapy. Cancer Treat Rev. 1988 Dec;15(4):279–292. doi: 10.1016/0305-7372(88)90026-6. [DOI] [PubMed] [Google Scholar]
- Degan P., Montesano R., Wild C. P. Antibodies against 7-methyldeoxyguanosine: its detection in rat peripheral blood lymphocyte DNA and potential applications to molecular epidemiology. Cancer Res. 1988 Sep 15;48(18):5065–5070. [PubMed] [Google Scholar]
- Fong L. Y., Jensen D. E., Magee P. N. DNA methyl-adduct dosimetry and O6-alkylguanine-DNA alkyl transferase activity determinations in rat mammary carcinogenesis by procarbazine and N-methylnitrosourea. Carcinogenesis. 1990 Mar;11(3):411–417. doi: 10.1093/carcin/11.3.411. [DOI] [PubMed] [Google Scholar]
- Gibson N. W., Hartley J. A., LaFrance R. J., Vaughan K. Differential cytotoxicity and DNA-damaging effects produced in human cells of the Mer+ and Mer- phenotypes by a series of 1-aryl-3-alkyltriazenes. Cancer Res. 1986 Oct;46(10):4999–5003. [PubMed] [Google Scholar]
- Hall C. N., Badawi A. F., O'Connor P. J., Saffhill R. The detection of alkylation damage in the DNA of human gastrointestinal tissues. Br J Cancer. 1991 Jul;64(1):59–63. doi: 10.1038/bjc.1991.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward I. P., Parsons P. G. Comparison of virus reactivation, DNA base damage, and cell cycle effects in autologous human melanoma cells resistant to methylating agents. Cancer Res. 1984 Jan;44(1):55–58. [PubMed] [Google Scholar]
- Jelinek J., Kleibl K., Dexter T. M., Margison G. P. Transfection of murine multi-potent haemopoietic stem cells with an E. coli DNA alkyltransferase gene confers resistance to the toxic effects of alkylating agents. Carcinogenesis. 1988 Jan;9(1):81–87. doi: 10.1093/carcin/9.1.81. [DOI] [PubMed] [Google Scholar]
- Kaina B., Fritz G., Mitra S., Coquerelle T. Transfection and expression of human O6-methylguanine-DNA methyltransferase (MGMT) cDNA in Chinese hamster cells: the role of MGMT in protection against the genotoxic effects of alkylating agents. Carcinogenesis. 1991 Oct;12(10):1857–1867. doi: 10.1093/carcin/12.10.1857. [DOI] [PubMed] [Google Scholar]
- Kataoka H., Hall J., Karran P. Complementation of sensitivity to alkylating agents in Escherichia coli and Chinese hamster ovary cells by expression of a cloned bacterial DNA repair gene. EMBO J. 1986 Dec 1;5(12):3195–3200. doi: 10.1002/j.1460-2075.1986.tb04629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleihues P., Kolar G. F., Margison G. P. Interaction of the carcinogen 3,3-dimethyl-1-phenyltriazene with nucleic acids of various rat tissues and the effect of a protein-free diet. Cancer Res. 1976 Jul;36(7 Pt 1):2189–2193. [PubMed] [Google Scholar]
- Kyrtopoulos S. A., Ampatzi P., Davaris P., Haritopoulos N., Golematis B. Studies in gastric carcinogenesis. IV. O6-methylguanine and its repair in normal and atrophic biopsy specimens of human gastric mucosa. Correlation of O6-alkylguanine-DNA alkyltransferase activities in gastric mucosa and circulating lymphocytes. Carcinogenesis. 1990 Mar;11(3):431–436. doi: 10.1093/carcin/11.3.431. [DOI] [PubMed] [Google Scholar]
- Lee S. M., Thatcher N., Dougal M., Margison G. P. Dosage and cycle effects of dacarbazine (DTIC) and fotemustine on O6-alkylguanine-DNA alkyltransferase in human peripheral blood mononuclear cells. Br J Cancer. 1993 Feb;67(2):216–221. doi: 10.1038/bjc.1993.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. M., Thatcher N., Margison G. P. O6-alkylguanine-DNA alkyltransferase depletion and regeneration in human peripheral lymphocytes following dacarbazine and fotemustine. Cancer Res. 1991 Jan 15;51(2):619–623. [PubMed] [Google Scholar]
- Lunn J. M., Harris A. L. Cytotoxicity of 5-(3-methyl-1-triazeno)imidazole-4-carboxamide (MTIC) on Mer+, Mer+Rem- and Mer- cell lines: differential potentiation by 3-acetamidobenzamide. Br J Cancer. 1988 Jan;57(1):54–58. doi: 10.1038/bjc.1988.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meer L., Janzer R. C., Kleihues P., Kolar G. F. In vivo metabolism and reaction with DNA of the cytostatic agent, 5-(3,3-dimethyl-1-triazeno)imidazole-4-carboxamide (DTIC). Biochem Pharmacol. 1986 Oct 1;35(19):3243–3247. doi: 10.1016/0006-2952(86)90419-3. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Mammalian O6-alkylguanine-DNA alkyltransferase: regulation and importance in response to alkylating carcinogenic and therapeutic agents. Cancer Res. 1990 Oct 1;50(19):6119–6129. [PubMed] [Google Scholar]
- Samson L., Derfler B., Waldstein E. A. Suppression of human DNA alkylation-repair defects by Escherichia coli DNA-repair genes. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5607–5610. doi: 10.1073/pnas.83.15.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souliotis V. L., Kaila S., Boussiotis V. A., Pangalis G. A., Kyrtopoulos S. A. Accumulation of O6-methylguanine in human blood leukocyte DNA during exposure to procarbazine and its relationships with dose and repair. Cancer Res. 1990 May 1;50(9):2759–2764. [PubMed] [Google Scholar]
- Walles S. A., Ringborg U. Induction and time course of DNA single-strand breaks in lymphocytes from patients treated with dacarbazine. Carcinogenesis. 1991 Jun;12(6):1153–1154. doi: 10.1093/carcin/12.6.1153. [DOI] [PubMed] [Google Scholar]
- Wild C. P., Smart G., Saffhill R., Boyle J. M. Radioimmunoassay of O6-methyldeoxyguanosine in DNA of cells alkylated in vitro and in vivo. Carcinogenesis. 1983 Dec;4(12):1605–1609. doi: 10.1093/carcin/4.12.1605. [DOI] [PubMed] [Google Scholar]
- van Delft J. H., van den Ende A. M., Keizer H. J., Ouwerkerk J., Baan R. A. Determination of N7-methylguanine in DNA of white blood cells from cancer patients treated with dacarbazine. Carcinogenesis. 1992 Jul;13(7):1257–1259. doi: 10.1093/carcin/13.7.1257. [DOI] [PubMed] [Google Scholar]


