Abstract
Zinc(II) phthalocyanine, a hydrophobic photosensitiser, was incorporated in unilamellar liposomes and studied in vivo for fluorescence kinetics and photodynamic activity. An observation chamber mounted in a dorsal skinfold of female WAG/Rij rats was used as a model system. In the chamber, an isogeneic mammary carcinoma was transplanted in the subcutaneous tissue. Phthalocyanine fluorescence was excited at 610 nm with a power density of 0.25 mW cm-2 and was detected above 665 nm through a high-pass filter using a two-stage image intensifier coupled to a charge-coupled device (CCD) camera. Following i.v. administration of 0.14 mg kg-1 of the drug, the fluorescence pharmacokinetics of the dye in vasculature, normal tissue and tumour tissue was determined as a function of time. Tumour fluorescence increased slowly to a maximum about 3 h post injection (p.i.), and remained well above the normal tissue fluorescence till 24 h p.i. Fluorescence in the circulation was always stronger than in the tissues. A treatment light dose at a wavelength of 675 nm was delivered 24 h p.i. One group of six animals received a total light dose of 150 J cm-2 (100 mW cm-2). A second group of six animals received a total light dose of 450 J cm-2 at the same dose rate. Vascular damage resulting from treatment was observed only at the final stages of the irradiation, despite the relatively high levels of fluorescence in the circulation. Immediate post-treatment (re)transplantation of the content of the chamber into the flank always resulted in tumour regrowth, confirming the presence of viable tumour cells following photodynamic therapy (PDT). When the chamber was left intact, the light dose of 450 J cm-2 yielded complete tissue necrosis. The role of the dye-carrier complex in shielding the vascular surrounding from photoproducts was studied in a third group of animals. The presence of peroxides was demonstrated in the serum of these animals after PDT with zinc phthalocyanine in liposomes (ZnPc-lip) using a total light dose of 450 J cm-2. This ex vivo observation supports the previously reported observations in vitro that the carrier complex is able to quench the photoproducts resulting from photoactivation of the photosensitiser which is present in the circulation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg K., Bommer J. C., Moan J. Evaluation of sulfonated aluminum phthalocyanines for use in photochemotherapy. Cellular uptake studies. Cancer Lett. 1989 Jan;44(1):7–15. doi: 10.1016/0304-3835(89)90101-8. [DOI] [PubMed] [Google Scholar]
- Bown S. G., Tralau C. J., Smith P. D., Akdemir D., Wieman T. J. Photodynamic therapy with porphyrin and phthalocyanine sensitisation: quantitative studies in normal rat liver. Br J Cancer. 1986 Jul;54(1):43–52. doi: 10.1038/bjc.1986.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasseur N., Ali H., Langlois R., Wagner J. R., Rousseau J., van Lier J. E. Biological activities of phthalocyanines--V. Photodynamic therapy of EMT-6 mammary tumors in mice with sulfonated phthalocyanines. Photochem Photobiol. 1987 May;45(5):581–586. doi: 10.1111/j.1751-1097.1987.tb07383.x. [DOI] [PubMed] [Google Scholar]
- Brasseur N., Ali H., Langlois R., van Lier J. E. Biological activities of phthalocyanines--IX. Photosensitization of V-79 Chinese hamster cells and EMT-6 mouse mammary tumor by selectively sulfonated zinc phthalocyanines. Photochem Photobiol. 1988 May;47(5):705–711. doi: 10.1111/j.1751-1097.1988.tb02768.x. [DOI] [PubMed] [Google Scholar]
- Candide C., Reyftmann J. P., Santus R., Mazière J. C., Morlière P., Goldstein S. Modification of epsilon-amino group of lysines, cholesterol oxidation and oxidized lipid-apoprotein cross-link formation by porphyrin-photosensitized oxidation of human low density lipoproteins. Photochem Photobiol. 1988 Aug;48(2):137–146. doi: 10.1111/j.1751-1097.1988.tb02798.x. [DOI] [PubMed] [Google Scholar]
- Chan W. S., Marshall J. F., Svensen R., Bedwell J., Hart I. R. Effect of sulfonation on the cell and tissue distribution of the photosensitizer aluminum phthalocyanine. Cancer Res. 1990 Aug 1;50(15):4533–4538. [PubMed] [Google Scholar]
- Evensen S. A., Galdal K. S., Nilsen E. LDL-induced cytotoxicity and its inhibition by anti-oxidant treatment in cultured human endothelial cells and fibroblasts. Atherosclerosis. 1983 Oct;49(1):23–30. doi: 10.1016/0021-9150(83)90004-7. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jori G., Tomio L., Reddi E., Rossi E., Corti L., Zorat P. L., Calzavara F. Preferential delivery of liposome-incorporated porphyrins to neoplastic cells in tumour-bearing rats. Br J Cancer. 1983 Aug;48(2):307–309. doi: 10.1038/bjc.1983.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRobert A. J., Bown S. G., Phillips D. What are the ideal photoproperties for a sensitizer? Ciba Found Symp. 1989;146:4–16. doi: 10.1002/9780470513842.ch2. [DOI] [PubMed] [Google Scholar]
- Maziere J. C., Santus R., Morliere P., Reyftmann J. P., Candide C., Mora L., Salmon S., Maziere C., Gatt S., Dubertret L. Cellular uptake and photosensitizing properties of anticancer porphyrins in cell membranes and low and high density lipoproteins. J Photochem Photobiol B. 1990 Jun;6(1-2):61–68. doi: 10.1016/1011-1344(90)85074-7. [DOI] [PubMed] [Google Scholar]
- Milanesi C., Biolo R., Reddi E., Jori G. Ultrastructural studies on the mechanism of the photodynamic therapy of tumors. Photochem Photobiol. 1987 Nov;46(5):675–681. doi: 10.1111/j.1751-1097.1987.tb04831.x. [DOI] [PubMed] [Google Scholar]
- Reddi E., Lo Castro G., Biolo R., Jori G. Pharmacokinetic studies with zinc(II)-phthalocyanine in tumour-bearing mice. Br J Cancer. 1987 Nov;56(5):597–600. doi: 10.1038/bjc.1987.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi E., Zhou C., Biolo R., Menegaldo E., Jori G. Liposome- or LDL-administered Zn (II)-phthalocyanine as a photodynamic agent for tumours. I. Pharmacokinetic properties and phototherapeutic efficiency. Br J Cancer. 1990 Mar;61(3):407–411. doi: 10.1038/bjc.1990.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal I. Phthalocyanines as photodynamic sensitizers. Photochem Photobiol. 1991 Jun;53(6):859–870. doi: 10.1111/j.1751-1097.1991.tb09900.x. [DOI] [PubMed] [Google Scholar]
- Star W. M., Marijnissen H. P., van den Berg-Blok A. E., Versteeg J. A., Franken K. A., Reinhold H. S. Destruction of rat mammary tumor and normal tissue microcirculation by hematoporphyrin derivative photoradiation observed in vivo in sandwich observation chambers. Cancer Res. 1986 May;46(5):2532–2540. [PubMed] [Google Scholar]
- Tralau C. J., Young A. R., Walker N. P., Vernon D. I., MacRobert A. J., Brown S. B., Bown S. G. Mouse skin photosensitivity with dihaematoporphyrin ether (DHE) and aluminium sulphonated phthalocyanine (AlSPc): a comparative study. Photochem Photobiol. 1989 Mar;49(3):305–312. doi: 10.1111/j.1751-1097.1989.tb04111.x. [DOI] [PubMed] [Google Scholar]
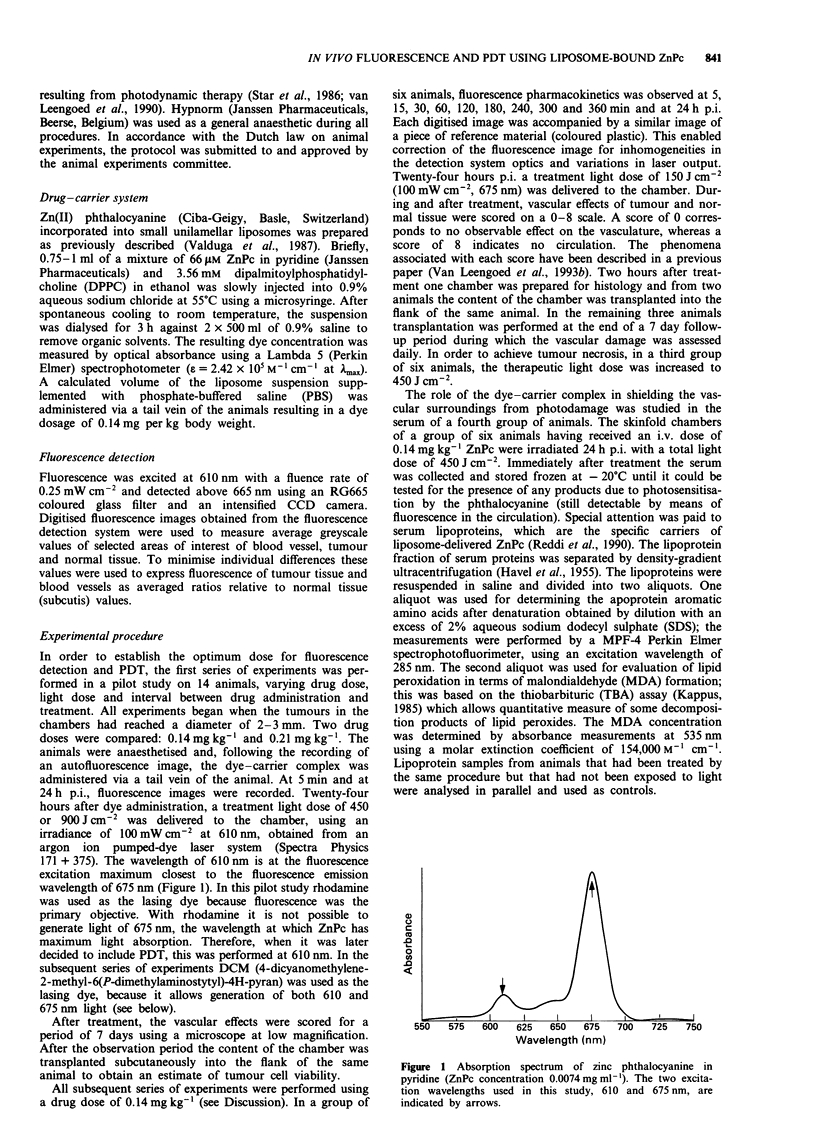
- Valduga G., Reddi E., Jori G. Spectroscopic studies on Zn(II)-phthalocyanine in homogeneous and microheterogeneous systems. J Inorg Biochem. 1987 Jan;29(1):59–65. doi: 10.1016/0162-0134(87)80012-0. [DOI] [PubMed] [Google Scholar]
- Weinstein J. N., Yoshikami S., Henkart P., Blumenthal R., Hagins W. A. Liposome-cell interaction: transfer and intracellular release of a trapped fluorescent marker. Science. 1977 Feb 4;195(4277):489–492. doi: 10.1126/science.835007. [DOI] [PubMed] [Google Scholar]
- Zhou C. N., Milanesi C., Jori G. An ultrastructural comparative evaluation of tumors photosensitized by porphyrins administered in aqueous solution, bound to liposomes or to lipoproteins. Photochem Photobiol. 1988 Oct;48(4):487–492. doi: 10.1111/j.1751-1097.1988.tb02850.x. [DOI] [PubMed] [Google Scholar]
- van Leengoed E., Versteeg J., van der Veen N., van den Berg-Blok A., Marijnissen H., Star W. Tissue-localizing properties of some photosensitizers studied by in vivo fluorescence imaging. J Photochem Photobiol B. 1990 Jun;6(1-2):111–119. doi: 10.1016/1011-1344(90)85080-g. [DOI] [PubMed] [Google Scholar]
- van Leengoed H. L., van der Veen N., Versteeg A. A., Ouellet R., van Lier J. E., Star W. M. In vivo fluorescence kinetics of phthalocyanines in a skin-fold observation chamber model: role of central metal ion and degree of sulfonation. Photochem Photobiol. 1993 Aug;58(2):233–237. doi: 10.1111/j.1751-1097.1993.tb09554.x. [DOI] [PubMed] [Google Scholar]
- van Leengoed H. L., van der Veen N., Versteeg A. A., Ouellet R., van Lier J. E., Star W. M. In vivo photodynamic effects of phthalocyanines in a skin-fold observation chamber model: role of central metal ion and degree of sulfonation. Photochem Photobiol. 1993 Oct;58(4):575–580. doi: 10.1111/j.1751-1097.1993.tb04935.x. [DOI] [PubMed] [Google Scholar]