Abstract
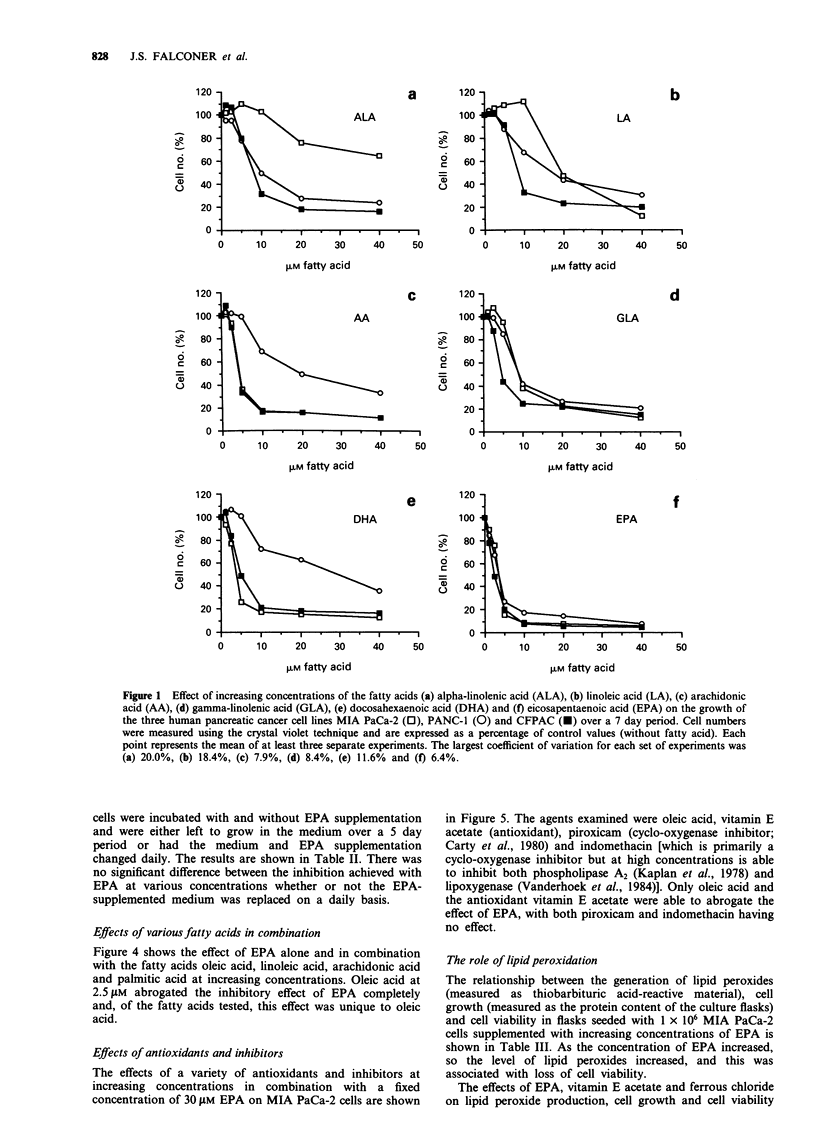
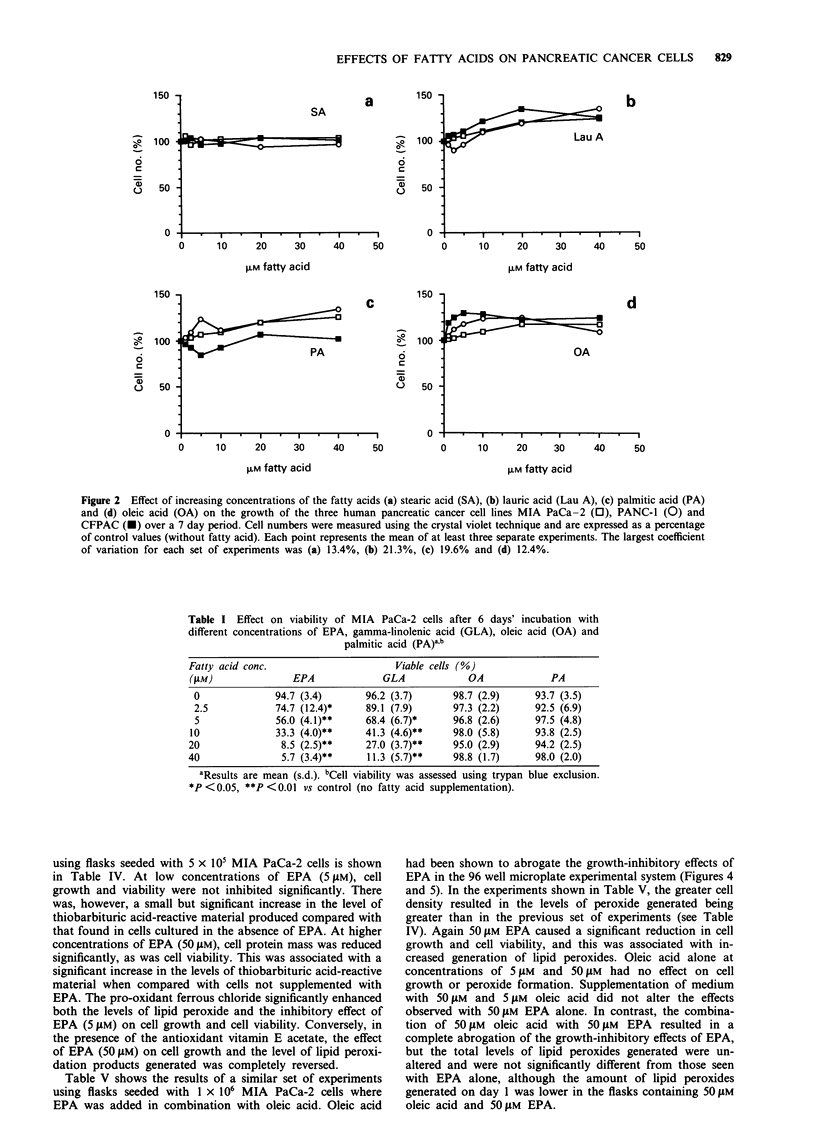
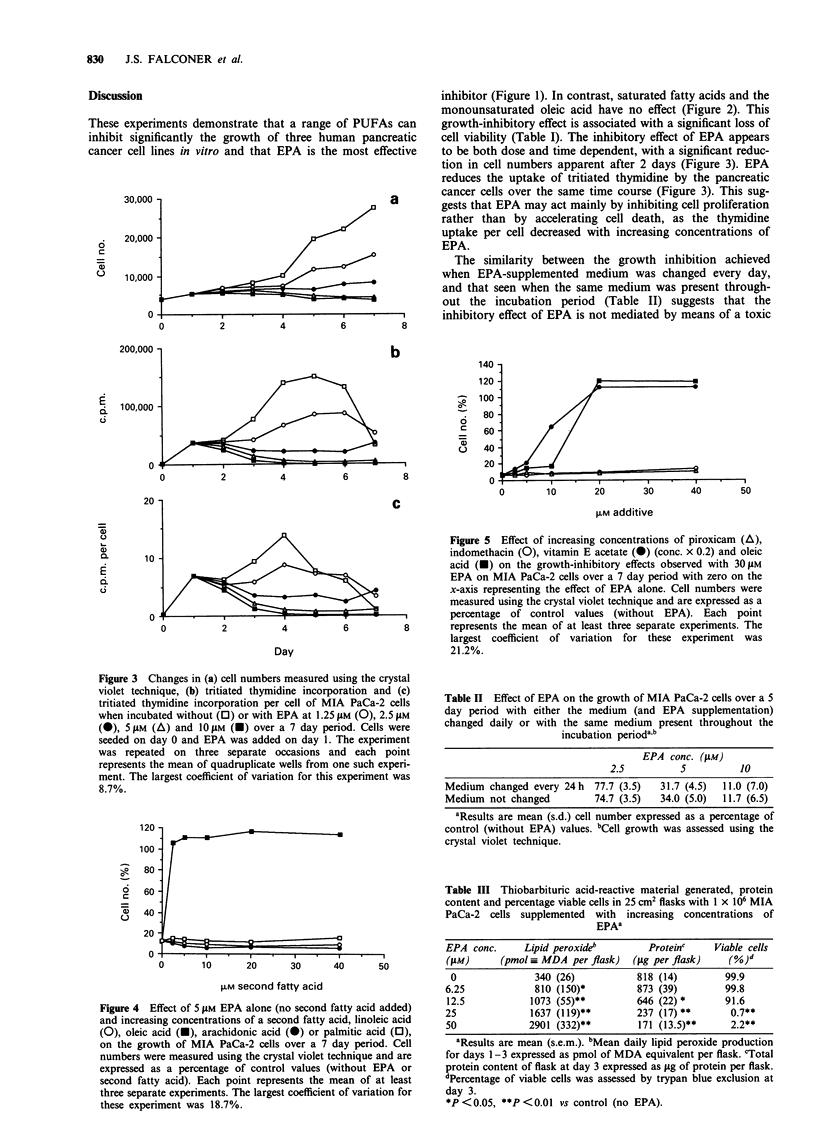
A number of polyunsaturated fatty acids have been shown to inhibit the growth of malignant cells in vitro. To investigate whether fatty acids modify the growth of human pancreatic cancer, lauric, stearic, palmitic, oleic, linoleic, alpha-linolenic, gamma-linolenic, arachidonic, docosahexaenoic and eicosapentaenoic (EPA) acids were each incubated with the cells lines MIA PaCa-2, PANC-1 and CFPAC at concentrations ranging from 1.25 microM to 50 microM and the effect of each fatty acid on cell growth was examined. All the polyunsaturated fatty acids tested had an inhibitory effect, with EPA being the most potent (ID50 2.5-5 microM). Monounsaturated or saturated fatty acids were not inhibitory. The action of EPA could be reversed with the anti-oxidant vitamin E acetate or with oleic acid. The cyclo-oxygenase inhibitors indomethacin and piroxicam had no effect on the action of EPA. The action of EPA appeared to be associated with the generation of lipid peroxides, although the level of lipid peroxidation did not always appear to correlate directly with the extent of cell death. The ability of certain fatty acids to inhibit significantly the growth of three human pancreatic cancer cell lines in vitro at concentrations which could be achieved in vivo suggests that administration of such fatty acids may be of therapeutic benefit in patients with pancreatic cancer.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck S. A., Smith K. L., Tisdale M. J. Anticachectic and antitumor effect of eicosapentaenoic acid and its effect on protein turnover. Cancer Res. 1991 Nov 15;51(22):6089–6093. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bronsgeest-Schoute H. C., van Gent C. M., Luten J. B., Ruiter A. The effect of various intakes of omega 3 fatty acids on the blood lipid composition in healthy human subjects. Am J Clin Nutr. 1981 Sep;34(9):1752–1757. doi: 10.1093/ajcn/34.9.1752. [DOI] [PubMed] [Google Scholar]
- Bégin M. E., Das U. N., Ells G., Horrobin D. F. Selective killing of human cancer cells by polyunsaturated fatty acids. Prostaglandins Leukot Med. 1985 Aug;19(2):177–186. doi: 10.1016/0262-1746(85)90084-8. [DOI] [PubMed] [Google Scholar]
- Bégin M. E., Ells G., Das U. N., Horrobin D. F. Differential killing of human carcinoma cells supplemented with n-3 and n-6 polyunsaturated fatty acids. J Natl Cancer Inst. 1986 Nov;77(5):1053–1062. [PubMed] [Google Scholar]
- Bégin M. E., Ells G., Horrobin D. F. Polyunsaturated fatty acid-induced cytotoxicity against tumor cells and its relationship to lipid peroxidation. J Natl Cancer Inst. 1988 Apr 6;80(3):188–194. doi: 10.1093/jnci/80.3.188. [DOI] [PubMed] [Google Scholar]
- Calman K. C. Malignancy. Cancer cachexia. Br J Hosp Med. 1982 Jan;27(1):28-9, 33-4. [PubMed] [Google Scholar]
- Canuto R. A., Muzio G., Biocca M. E., Dianzani M. U. Lipid peroxidation in rat AH-130 hepatoma cells enriched in vitro with arachidonic acid. Cancer Res. 1991 Sep 1;51(17):4603–4608. [PubMed] [Google Scholar]
- Carty T. J., Stevens J. S., Lombardino J. G., Parry M. J., Randall M. J. Piroxicam, a structurally novel anti-inflammatory compound. Mode of prostaglandin synthesis inhibition. Prostaglandins. 1980 May;19(5):671–682. doi: 10.1016/0090-6980(80)90166-5. [DOI] [PubMed] [Google Scholar]
- Diplock A. T., Balasubramanian K. A., Manohar M., Mathan V. I., Ashton D. Purification and chemical characterisation of the inhibitor of lipid peroxidation from intestinal mucosa. Biochim Biophys Acta. 1988 Sep 2;962(1):42–50. doi: 10.1016/0005-2760(88)90093-8. [DOI] [PubMed] [Google Scholar]
- Dippenaar N., Booyens J., Fabbri D., Katzeff I. E. The reversibility of cancer: evidence that malignancy in melanoma cells is gamma-linolenic acid deficiency-dependent. S Afr Med J. 1982 Oct 2;62(15):505–509. [PubMed] [Google Scholar]
- Fujiwara F., Todo S., Imashuku S. Antitumor effect of gamma-linolenic acid on cultured human neuroblastoma cells. Prostaglandins Leukot Med. 1986 Aug;23(2-3):311–320. doi: 10.1016/0262-1746(86)90198-8. [DOI] [PubMed] [Google Scholar]
- Gavino V. C., Miller J. S., Ikharebha S. O., Milo G. E., Cornwell D. G. Effect of polyunsaturated fatty acids and antioxidants on lipid peroxidation in tissue cultures. J Lipid Res. 1981 Jul;22(5):763–769. [PubMed] [Google Scholar]
- Hudson E. A., Beck S. A., Tisdale M. J. Kinetics of the inhibition of tumour growth in mice by eicosapentaenoic acid-reversal by linoleic acid. Biochem Pharmacol. 1993 Jun 9;45(11):2189–2194. doi: 10.1016/0006-2952(93)90188-3. [DOI] [PubMed] [Google Scholar]
- Kaplan L., Weiss J., Elsbach P. Low concentrations of indomethacin inhibit phospholipase A2 of rabbit polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2955–2958. doi: 10.1073/pnas.75.6.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmali R. A., Marsh J., Fuchs C. Effect of omega-3 fatty acids on growth of a rat mammary tumor. J Natl Cancer Inst. 1984 Aug;73(2):457–461. doi: 10.1093/jnci/73.2.457. [DOI] [PubMed] [Google Scholar]
- Karmali R. A., Reichel P., Cohen L. A., Terano T., Hirai A., Tamura Y., Yoshida S. The effects of dietary omega-3 fatty acids on the DU-145 transplantable human prostatic tumor. Anticancer Res. 1987 Nov-Dec;7(6):1173–1179. [PubMed] [Google Scholar]
- Mahoney E. M., Hamill A. L., Scott W. A., Cohn Z. A. Response of endocytosis to altered fatty acyl composition of macrophage phospholipids. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4895–4899. doi: 10.1073/pnas.74.11.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara N., Fuchimoto S., Orita K. Antiproliferative effects of natural human tumor necrosis factor-alpha, interferon-alpha, and interferon-gamma on human pancreatic carcinoma cell lines. Int J Pancreatol. 1991 Apr;8(3):235–243. doi: 10.1007/BF02924542. [DOI] [PubMed] [Google Scholar]
- Pritchard G. A., Jones D. L., Mansel R. E. Lipids in breast carcinogenesis. Br J Surg. 1989 Oct;76(10):1069–1073. doi: 10.1002/bjs.1800761028. [DOI] [PubMed] [Google Scholar]
- Thorngren M., Nilsson E., Gustafson A. Plasma lipoproteins and fatty acid composition during a moderate eicosapentaenoic acid diet. Acta Med Scand. 1986;219(1):23–28. doi: 10.1111/j.0954-6820.1986.tb03271.x. [DOI] [PubMed] [Google Scholar]
- Vanderhoek J. Y., Ekborg S. L., Bailey J. M. Nonsteroidal anti-inflammatory drugs stimulate 15-lipoxygenase/leukotriene pathway in human polymorphonuclear leukocytes. J Allergy Clin Immunol. 1984 Sep;74(3 Pt 2):412–417. doi: 10.1016/0091-6749(84)90140-4. [DOI] [PubMed] [Google Scholar]
- Wicha M. S., Liotta L. A., Kidwell W. R. Effects of free fatty acids on the growth of normal and neoplastic rat mammary epithelial cells. Cancer Res. 1979 Feb;39(2 Pt 1):426–435. [PubMed] [Google Scholar]
- Williamson R. C. Pancreatic cancer: the greatest oncological challenge. Br Med J (Clin Res Ed) 1988 Feb 13;296(6620):445–446. doi: 10.1136/bmj.296.6620.445. [DOI] [PMC free article] [PubMed] [Google Scholar]


