Abstract
There is an urgent need for new antimicrobial therapies to combat drug resistance, new pathogens, and the relative inefficacy of current therapy in compromised hosts. Ionizing radiation can kill microorganisms quickly and efficiently, but this modality has not been exploited as a therapeutic antimicrobial strategy. We have developed methods to target ionizing radiation to a fungal cell by labeling a specific mAb with the therapeutic radioisotopes Rhenium-188 and Bismuth-213. Radiolabeled antibody killed cells of human pathogenic fungus Cryptococcus neoformans in vitro, thus converting an antibody with no inherent antifungal activity into a microbicidal molecule. Administration of radiolabeled antibody to mice with C. neoformans infection delivered 213Bi and 188Re to the sites of infection, reduced their organ fungal burden, and significantly prolonged their survival without apparent toxicity. This study establishes the principle that targeted radiation can be used for the therapy of an infectious disease, and suggests that it may have wide applicability as an antimicrobial strategy.
Radiation possesses microbicidal properties and γ-irradiation is routinely used for the sterilization of medical supplies and certain foods. Ionizing radiation such as γ-rays, β-particles, and especially α-particles from external sources can kill different strains of bacteria and fungi such as Escherichia coli, Cryptococcus neoformans (CN), and Mycobacterium tuberculosis (1–3). Despite its microbicidal properties, radiation is not used in current antimicrobial therapy. Dadachova (4) recently proposed using therapeutic radionuclides for the treatment of multidrug-resistant infections. However, to realize the full benefits of ionizing radiation as an anti-infective treatment, it is important that the radiation be specifically targeted to the sites of infection to minimize toxicity to the host. Evidence that radiation can be targeted to a focus of infection by specific antibody comes from the observation that radiolabeled antibodies can be used to visualize the sites of infection in patients with Pneumocystis carinii pneumonia (5) and tuberculomas in rabbits infected with Mycobacterium bovis (bacillus Calmette–Guérin) (6). The use of antibodies to granulocytes for imaging the sites of infection in patients has also been reported (7).
The fact that radiolabeled antibodies can detect foci of infection in vivo (5, 6) implies that antigen–antibody interactions can be used to deliver radionuclides to microorganisms in vivo. Hence, the next logical step in developing radiotherapy for infectious diseases would be to ascertain whether particulate radiation could be targeted to kill microorganisms in the infectious foci by specific radiolabeled antibodies. This option is potentially very attractive as an antifungal strategy, because fungal cells in tissue are relatively large targets with dimensions that approximate the size of host cells. Furthermore, in contrast to tumor cells, yeast cells are antigenically very different from host tissue, and thus provide the potential for abundant antigen–antibody interactions with the fungal cell and low crossreaction with the host tissue. Targeting fungi with radioimmunotherapy (RIT) makes sense, because fungal diseases are notoriously difficult to treat and currently constitute a major clinical problem (8). Despite its theoretical promise, we are not aware of attempts to explore the potential usefulness of RIT against infectious diseases.
In this study, we explored the potential efficacy of RIT against an experimental fungal infection by using CN. CN is a major fungal pathogen that causes life-threatening meningoencephalitis in 6–8% of patients with AIDS. Cryptococcal infections in immunocompromised patients are often incurable, because antifungal drugs do not eradicate the infection in the setting of severe immune dysfunction (9, 10). CN provides a good system to study the potential usefulness of RIT, because there are excellent mouse models available, well characterized mAbs to CN antigens exist, and immunotherapy of CN infection with passive antibody (11) is already in clinical evaluation.
Two radioisotopes were evaluated in this study: a high-energy β-emitter Rhenium-188 (188Re) and an α-particle emitter Bismuth-213 (213Bi). 188Re (t[1/2] = 16.9 h) (Emax = 2.12 MeV) has recently emerged as an attractive therapeutic radionuclide for cancer radioimmunotherapy, palliation of skeletal bone pain, and endovascular brachytherapy to prevent restenosis after angioplasty (12–14). 213Bi (t[1/2] = 45.6 min) emits a high linear energy transfer α-particle with E = 5.9 MeV with a path length in tissue of 50–80 μm. Theoretically, a cell can be killed with one or two α-particle hits. 213Bi was proposed for use in single-cell disorders and some solid cancers (15–18), and has been used to treat patients with leukemia in Phase I clinical trials (19, 20).
Methods
CN. American Type Culture Collection strain 24067 (serotype D) was used in all experiments. It was grown in Sabouraud dextrose broth (Difco) for 24 h at 30°C with constant shaking. Organisms were centrifuged three times with PBS, pH 7.2, at 200 × g.
Antibodies and Immunoreactivity Determination. MAb 18B7 (IgG1) was produced as described (21). MAb MOPC21 (ICN Biomedicals, Aurora, OH) was used as an irrelevant isotype-matched control mAb in therapy experiments. The immunoreactivity of 18B7 mAb radiolabeled with 111In or 99mTc was determined by ELISA as described in ref. 22. Experiments were performed in triplicate.
Conjugation of Bifunctional Chelate SCN-CHXA″ to Antibodies. mAbs 18B7 and MOPC21 were conjugated to N-[2-amino-3-(p-isothiocyanatophenyl)propy1]-trans-cyclohexane-1,2-diamine-N, N′,N″,N′,N″″-pentaacetic acid (CHXA″) as in ref. 23, with an average number of chelates per antibody ranging from 0.7 to 3.0 as determined by the yttrium-arsenazo III spectrophotometric method (24).
Radioisotopes. Na99mTcO4 was purchased from Syncor (Bronx, NY); 111InC13 was purchased from Iso-Tex Diagnostics, Friends-wood, TX; 188Re in the form of Na perrhenate Na188ReO4 was eluted from a 188W/188Re generator (Oak Ridge National Laboratory, Oak Ridge, TN); and Actinium-225 (225Ac) for construction of a 225Ac/213Bi generator (25) was acquired from Oak Ridge National Laboratory.
Radiolabeling of 18B7 and MOPC21 Antibodies. The widely accepted nuclear medicine practice of “matching pairs” of radiopharmaceuticals uses diagnostic isotopes (no α- or β-particle emissions), with chemistries similar to those of therapeutic isotopes for labeling proteins, imaging procedures, and quality control (26). 99mTc is “a matching pair” isotope for 188Re (27) and 111In is a matching pair isotope for 213Bi (28).
MAbs 18B7 and MOPC21 were directly labeled with either 99mTc or 188Re through reduction of antibody disulfide bonds with DTT (29).
CHXA″ 18B7 was radiolabeled with 213Bi and 111In, as described in refs. 25 and 30, respectively.
Serum Stability of Radiolabeled 18B7. 99mTc-18B7, 188Re-18B7, and 111In-CHXA-″ 18B7 were incubated in human serum or murine serum for 24 h, and analyzed on a size-exclusion HPLC column.
Biodistribution of 99mTc-18B7 and 99mTc-MOPC21 in CN-Infected BALB/c Mice. Six- to 8-week-old female BALB/c mice were infected intratracheally as described (31). The intratracheal model was used because it initially causes a discrete lung infection, facilitating imaging of the infectious process using localized labeled antibody.
Mice were killed with a mixture of 125 mg/kg ketamine and 10 mg/kg xylazine, and were inoculated with 106 CN cells into the trachea. On the 5th day after infection, three mice were pretreated with 1.0 mg of 18B7 to bind the excess of capsular polysaccharide (CPS) in circulation. One hour later, three infected and three infected/pretreated mice were i.v. injected with 0.125 mCi (0.05 mg; 1 Ci = 37 GBq) of 99mTc-18B7, and the other three infected mice were injected with the irrelevant IgG1 control 99mTc-MOPC21. Three healthy mice were injected with the same amount of radiolabeled conjugate and served as controls. At 24 h, mice were killed, and their major organs were removed, weighed, and counted in a γ-counter (Wallac, Turku, Finland).
Biodistribution and Scintigraphic Imaging of 188Re-18B7 and 188Re-MOPC21 in Systemically Infected with CN A/JCr Mice. Nine groups of three A/JCr female mice (National Cancer Institute, Frederick, MD) were used. Groups 1–6 were i.v. infected with 105 CN cells, and groups 7–9 served as healthy controls. Approximately 24 h after infection, groups 1–3 received i.p. injections of 20 μCi of 188Re-18B7, groups 4–6 received 20 μCi of 188Re-MOPC21, and groups 7–9 of healthy mice received 20 μCi of 188Re-18B7. At 24, 48, and 72 h after injection of radioactivity, the mice were anesthetized with Isoflurane and were scintigraphically imaged with an eZ-SCOPE handheld semiconductor γ-camera (Anzai Medical, Tokyo). After imaging, the mice were killed, their major organs were removed, weighed, and counted in a γ-counter.
Determination of CPS Antigens Levels in the Blood of Infected Mice. The blood of the A/JCr mice (200 μl) i.v. infected 24 h before the analysis was collected by retroorbital bleeding, allowed to coagulate, and the serum was then separated by centrifugation. A volume of 60 μl of serum was analyzed for the presence of the CN CPS antigens by using a latex-crypto antigen detection system (IMMY, Immuno-Mycologics, Norman, OK), which has a sensitivity of 12 ng/ml for CPS antigens.
In Vitro Activity of 188Re-Radiolabeled and 213Bi-Radiolabeled Antibodies Against CN. CN (105 cells) was placed in microcentrifuge tubes in 0.1 ml of PBS. Serially diluted 188Re-18B7, 188Re-MOPC21, 213Bi-CHXA″-18B7, or 213Bi-CHXA″-MOPC21 in 0.5 ml of PBS was added to obtain the desired concentrations of radioactivity. After 30-min incubation at 37°C under constant shaking, CN cells were collected by centrifugation, washed with PBS to remove nonbound radioactivity, suspended in 1 ml of PBS, and incubated at 4°C for 1 h or 48 h on a rocker for 213Bi-labeled or 188Re-labeled antibodies, respectively. After the incubation, 103 cells were removed from each tube, diluted with PBS, and plated to determine colony-forming units (cfu) (1 colony = 1 cfu). Experiments were performed in duplicate.
Treatment of A/JCr Mice Infected with CN with Radiolabeled mAbs. The efficacy of RIT against CN infection was tested in A/JCr mice. This mouse strain was selected because it is very susceptible to CN infections, presumably because of partial complement deficiency (32). Mice with partial complement deficiency succumb rapidly with disseminated infection when i.v. infected (33).
Nine groups of 10 A/JCr female mice were i.v. infected with 105 CN cells. The i.v. infection results in rapid death of infected mice, and is a standard model used for antifungal susceptibility testing (33). Twenty-four hours after infection, groups 1, 2, and 3 received i.p. injections of 50, 100, and 200 μCi of 213Bi-CHXA″-18B7, respectively; group 4 received i.p. injections of 100 μCi of 213Bi-CHXA″-MOPC21; groups 5, 6, and 7 received i.p. injections of 50, 100, and 200 μCi of 188Re-18B7, respectively; and group 8 received i.p. injections of 100 μCi of 188Re-MOPC21. The amount of antibody per injection was 30–50 μg, and the injection volume was 0.2 ml of PBS. Group 9 received 0.1 ml of PBS, and group 10 received 50 μg of nonradioactive 18B7 in 0.1 ml of PBS. The mice were observed, and survival was recorded every 24 h for 75 days. Survival studies were performed twice. Some of the surviving mice were killed, and their lungs were analyzed for cfu.
Determination of Organ cfu. A group of 25 A/JCr female mice was i.v. infected with 105 CN cells. At 24 h after infection, the mice were separated into groups of five, and groups 1, 2, and 3 received i.p. injections of 50, 100, and 200 μCi of 188Re-18B7, respectively. The amount of antibody per injection was 30–50 μg in 0.2 ml of PBS. Group 4 received 50 μg of cold 18B7 in 0.1 ml of PBS, and control group 5 was left untreated. Forty-eight hours after treatment, three mice per group were killed, their lungs and brains were removed, blotted to remove excess blood, counted in a γ-counter, weighed, homogenized in PBS, and dilutions of the homogenate were plated to determine cfu.
Determination of Platelet Counts. For measurement of platelet counts, blood was collected from the tail vein of infected A/JCr mice into heparinized capillary tubes before therapy with 188Re-18B7 mAb, as well as 48 h, 6 days, and 13 days posttherapy. Platelets were counted in a hemocytometer by using phase contrast, at ×400 magnification, as described in ref. 34.
Histological Evaluation. For lung histology, the right upper lobe of the lung from the infected controls, and from the 188Re-18B7 mAb-treated A/JCr mice was removed at 48 h posttreatment, fixed in 10% formalin, embedded in paraffin, cut into 5-μm sections, and stained with hematoxylin/eosin.
Statistics. The Wilcoxon rank sum test was used to compare organs uptake in biodistribution studies. A Student's t test for unpaired data was used to analyze differences in the number of cfu between differently treated groups during in vitro therapy studies. The log-rank test was used to assess the course of mouse survival. Differences were considered statistically significant when P values were <0.05.
Results
Radiolabeling and Immunoreactivity of 18B7 mAb. In anticipation of RIT experiments, we evaluated various techniques for attaching 188Re, 99mTc, 213Bi, and 111In to 18B7 mAb. Direct labeling of 18B7 mAb with 99mTc and 188Re through generation of thiol groups produced 90 ± 5% and 87 ± 4% yields for 99mTc and 188Re, respectively. Radiolabeling yields for CHXA″-18B7 with both 111In and 213Bi were 90 ± 4%. Subsequent purification gave products with radiochemical purity of 97 ± 1%. Specific activities were 3.2 ± 0.5 mCi/mg for 99mTc-labeled or 188Re-labeled mAbs, and 0.3 ± 0.05 mCi/mg 99mTc-labeled or 188Re-labeled mAbs for 111In and 213Bi-labeled mAbs.
The mAb 18B7 proved to be a robust molecule that could be labeled with a variety of radioisotopes without a loss of immunoreactivity through either direct labeling or by using a bifunctional chelating agent, because >90% of radiolabeled 18B7 bound to glucuronoxylomannan antigen (see Fig. 4, which is published as supporting information on the PNAS web site, www.pnas.org). For CHXA″-18B7 conjugates, the 1.5 ligand per mAb molar ratio in the conjugation reaction resulted in 0.7–0.9 ligand per mAb molecule, which preserved its immunoreactivity.
Serum Stability of Radiolabeled 18B7. Negligible radioactivity was lost from the 99mTc-18B7 and 188Re-18B7 in the form of small molecular size radioactive species after incubation in either human or murine sera. 111In-CHXA″-18B7 did not release any radioactivity after serum incubation (results not shown). These data established that radiolabeled mAb 18B7 conjugates were stable in both human and murine sera.
Biodistribution of Radiolabeled 18B7 mAb in Locally Infected BALB/c Mice and in Systemically Infected A/JCr Mice. Radiolabeled antibody distribution in vivo was studied by using two different models of infection. The first study used intratracheal innoculation of CN into BALB/c mice, which results in a localized pulmonary infection that permits the evaluation of antibody targeting to an infected organ (Table 1). The uptake of 99mTc-18B7 in the blood, liver, kidney, and spleen in infected mice was higher than in infected mice pretreated with 1 mg of unlabeled mAb 18B7, in the control mice, or in infected mice injected with irrelevant control 99mTc-MOPC21 (P < 0.05). This finding may be due to formation of radiolabeled antibody–antigen complexes, which may have different clearance and metabolism patterns relative to antibody alone (35). The uptake in the lungs and the spleens of infected mice was two times higher than was observed in control mice or in infected/pretreated mice (P < 0.05), and almost four times higher than in mice given 99mTc-MOPC21. Interestingly, there were no significant differences in the lungs and spleen uptake of mice pretreated with unlabeled mAb 18B7 and controls (P = 0.8), possibly because of epitope blocking in the target site by unlabeled antibody. No significant release of 99mTc radiolabel occurred, as indicated by the low uptake in the stomach of 99mTc-MOPC21. By 24 h postinjection ≈10% initial dose per g was delivered to the lungs infected with CN, a dose that should be sufficient to deliver fungicidal levels of therapeutic radioisotopes to the infectious foci. The relatively high lung uptake (4–5%) of radiolabeled 18B7 in the pretreated and control mice may be due to uptake by pulmonary cells, including macrophages. These results establish that radiolabeled antibody will target an organ in CN-infected mice.
Table 1. Biodistribution of i.v. injected 99mTc-18B7 and 99mTc-MOPC21 antibodies at 24 h postinjection in mice intratracheally infected with CN and healthy BALB/c mice.
| Mice
|
||||
|---|---|---|---|---|
| Organ | Infected with CN, 99mTc-18B7 injection | Infected with CN, 99mTc-MOPC21 injection | Infected with CN and pretreated with 1 mg of nonradioactive 18B7 before 99mTc-18B7 injection | Healthy controls, 99mTc-18B7 injection |
| Blood | 12.77 (1.22) | 9.02 (2.13) | 8.25 (0.22) | 8.0 (0.70) |
| Liver | 8.71 (1.22) | 1.27 (0.34) | 6.71 (0.52) | 4.77 (0.32) |
| Kidney | 9.88 (0.21) | 3.07 (1.00) | 7.26 (0.28) | 4.47 (1.31) |
| Spleen | 4.86 (0.14) | 1.50 (0.09) | 2.53 (0.54) | 2.56 (0.62) |
| Stomach | 2.43 (0.08) | 1.04 (0.33) | 5.39 (1.32) | 1.57 (0.35) |
| Lungs | 10.27 (1.02) | 2.66 (0.50) | 4.57 (0.33) | 4.57 (0.59) |
Results were obtained from n = 3 mice per time point and were tabulated as percent initial dose per g. Numbers in parentheses are SD.
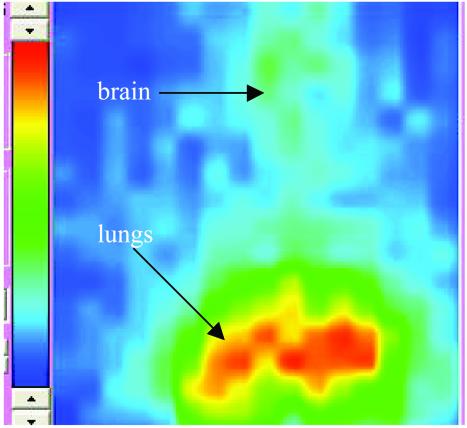
The second model used i.v. infection of A/JCr mice with CN. This model is the same as used in the RIT experiments (see below). Fig. 5 a–c, which is published as supporting information on the PNAS web site, shows the percent initial dose per g in CN-infected A/JCr mice in comparison with the healthy controls. At all three time intervals (24, 48, and 72 h), the uptake of radioactivity in the liver and spleen of infected mice given 188Re-18B7 was higher (P < 0.05) than in control groups, which is the consequence of the deposition of antibody–antigen complex (10). In contrast, the activity in the blood of noninfected mice was significantly higher than in infected mice (P < 0.05), which also resulted in higher uptake of 188Re-18B7 mAb in the brain of healthy mice. The uptake in the lungs of infected mice was twice that of healthy controls and 188Re-MOPC21-injected mice at 24 h and 48 h and equalized at 72 h, which can be attributed to the shedding of CPS antigen together with the bound antibody. The scintigraphic image (Fig. 1) of the infected mouse 48 h postinjection of 188Re-18B7 mAb obtained with the high-resolution γ-camera shows the uptake in the lungs and the brain of the mouse.
Fig. 1.
Immunoscintigraphic image (48 h) of infected A/JCr mouse, imaged with 188Re-18B7 mAb. The lungs and the brain are marked with arrows. On a color scale, red represents the highest radioactivity uptake.
CPS Antigen Levels in the Blood of Infected Mice. CPS antigens were found in the blood of infected mice at 24 h postinfection by using the latex-crypto antigen detection system at 1/80 and 1/160 dilutions corresponding to a serum CPS concentration of ≈1.9 μg/ml.
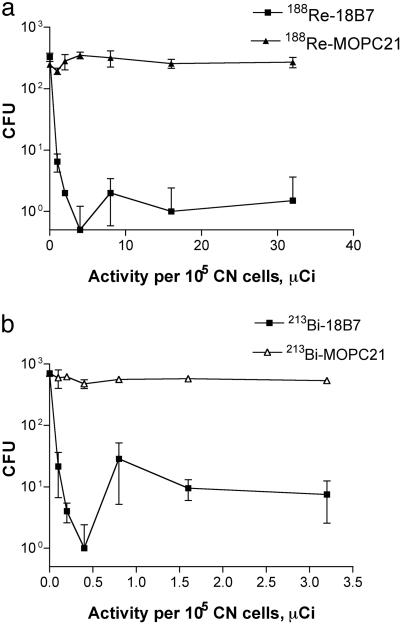
In Vitro Susceptibility of CN to 188Re-Radiolabeled and 213Bi-Radiolabeled Antibodies. For 188Re-18B7 incubated with 105 CN cells, the minimal inhibitory concentration was 4 μCi/1.25 μg 18B7 (Fig. 2). In contrast, the control antibody 188Re-MOPC21 with the same specific activity produced only minimal killing (P = 0.0008), presumably reflecting fungal damage from the radioactivity in solution. The significantly higher killing associated with the specific antibody almost certainly reflects higher radiation exposure for CN as a consequence of antibody binding to the CN capsule. The activity of 188Re-18B7 against CN in vitro indicated that radiolabeling made the antibody microbicidal.
Fig. 2.
The dose dependence of CN killing by radiolabeled mAbs. (a) 188Re-labeled mAbs. (b) 213Bi-labeled mAbs.
Because the relative biological effectiveness of α-particles is several times higher than that of β-particles (1), we used 1/10 of the radioactivity concentration in the incubation of CN with 213Bi-labeled antibodies than we used for the experiment with 188Re. The minimal inhibitory concentration for 213Bi-CHXA″-MOPC21 was 0.4 μCi/1.5 μg 18B7 mAb. The fungicidal activity of the irrelevant mAb 213Bi-CHXA″-MOPC21 was negligible (P = 0.0006) at the activity concentrations studied. This result attests to a very high killing efficiency of 213Bi toward CN, as high linear energy transfer of α-particles makes it possible to kill a cell with one to two hits (several hundred hits per cell are needed when β-emitting radionuclides are used). There was no statistically significant difference between cfu numbers at 0.5 μCi and at higher activities (P > 0.05), presumably reflecting a very low number of colonies at the most effective doses because of massive killing of yeast cells. The high efficiency of particulate radiation in killing CN cells is evident from the fact that levels of particulate radiation emitted by 188Re and 213Bi, which are efficient against mammalian cells (23), were also able to kill CN.
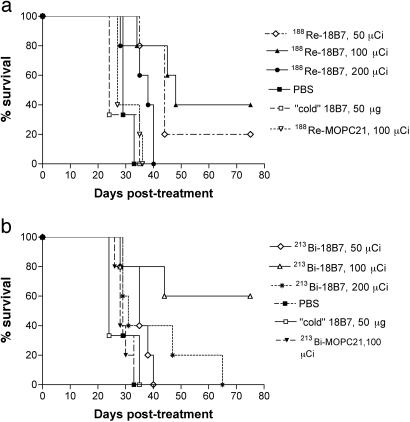
Treatment of A/JCr Mice Lethally Infected with CN with Radiolabeled mAbs. Mice treated with radiolabeled CN-specific mAb 18B7 lived significantly longer, on average, than mice given irrelevant labeled IgG1 or PBS (Fig. 3 and Table 3, which is published as supporting information on the PNAS web site). We used a labeled irrelevant mAb (213Bi-labeled or 188Re-labeled IgG1 MOPC21) to control for the possibility that Fc receptor binding by the radiolabeled IgG to phagocytes at the site of infection might result in nonspecific killing. Remarkably, on day 75 posttherapy, 60% of mice in 213Bi group were alive after treatment with 100 μCi of 213Bi-CHXA″-18B7 (P < 0.05). In the 188Re group, 40% and 20% of mice were alive after treatment with 100 (P < 0.005) and 50 μCi (P < 0.05) of 188Re-18B7, respectively. Mice infected with CN and given RIT had significantly reduced fungal burden in lungs and brains 48 h after treatment than did infected mice in the control groups (Table 2). Whereas there was no difference in the reduction of the fungal burden in the lungs between the groups that received 50 and 100 μCi of 188Re-18B7, treatment with 200 μCi of 188Re-18B7 significantly lowered lung cfu relative to the lower activities (P < 0.05). Hence, administration of CN-specific radiolabeled antibody prolonged survival and reduced organ fungal burden in infected mice.
Fig. 3.
Kaplan–Meier survival curves for A/JCr mice i.v. infected with 105 CN cells 24 h before treatment with 50–200 μCi of 188Re-labeled mAbs (a) and 50–200 μCi of 213Bi-labeled mAbs (b). Mice injected with PBS or 50 μg of nonradioactive 18B7 served as controls.
Table 2. CN CFUs in the lungs and the brains of A/JCr mice i.v. infected with 105 CN organisms and treated with 188Re-18B7 mAb 24 h after infection.
| No. of cfu/g of tissue, × 104
|
||||
|---|---|---|---|---|
| Organ | Untreated mice and treated with unlabeled 18B7, 50 μg | 50 μCi of 188Re-18B7 | 100 μCi of 188Re-18B7 | 200 μCi of 188Re-18B7 |
| Lungs | 550 (47)* | 41 (6) (P = 0.001) | 21 (5) (P = 0.001) | 3.3 (2) (P = 0.001) |
| Brains | 11 (5) | 0.10 (0.05) (P < 0.001) | 0.8 (0.1) (P = 0.002) | 1.1 (0.1) (P = 0.002) |
Mice were killed 48 h after the treatment with 188Re-18B7 (188Re; t1/2 = 2.8 d); the colony counts for 100 and 10 times dilutions for the lungs and brains, respectively, are given. Numbers in parentheses are SD.
P values were calculated by the Wilcoxon test, and the groups treated with 188Re-18B7 were compared with the combined group of untreated mice and those treated with unlabeled 18B7 mAb.
Survival of A/JCr mice was dose dependent for both 213Bi and 188Re radioisotopes: whereas 50 μCi of 213Bi-CHXA″-18B7 failed to produce any therapeutic effect, both the 100- and 200-μCi doses prolonged mouse survival (Fig. 3 and Table 3). Interestingly, the 200 μCi of 213Bi-CHXA″-18B7 dose was less efficient, possibly because of the fact that it may have approached the MTA (maximum tolerated activity) for this particular combination of antibody and radioisotope. In the 188Re group, a 50-μCi dose of 188Re-18B7 resulted in some prolongation of survival, a 100-μCi dose caused significant prolongation, and a 200-μCi dose was, apparently, too toxic, with all mice dying by day 40.
Low levels of cfu were detected in the lungs of surviving mice killed at the completion of survival studies.
Toxicity Studies. The number of platelets in peripheral blood was determined as a measure of radiation toxicity in CN-infected A/JCr mice treated with the higher (100 and 200 μCi) doses of 188Re-18B7 mAb, and in nontreated infected controls (Table 4, which is published as supporting information on the PNAS web site). Progression of CN infection resulted in reduced platelet numbers in nontreated mice. Mice treated with 188Re-18B7 had higher platelet counts than control mice on day 2 posttreatment, which could reflect the therapeutic effect of radiolabeled antibody on the underlying CN infection (confirmed by reduced fungal burden in the lungs and the brains). Platelet counts decreased significantly on day 6 posttreatment, with more pronounced decrease observed for the highest treatment dose of 200 μCi of 188Re-18B7. This result can be explained by the fact that the nadirs in platelet counts is usually reached at 1 week after radiolabeled antibody administration in RIT of cancer patients (16). Remarkably, on day 13 posttherapy, the platelet count in 100 μCi of 188Re-18B7 came back to normal, which reflects the therapeutic effect of this dose, whereas it remained the same in infected controls, and it further decreased in 200 μCi of 188Re-18B7 group, attesting to radiotoxicity of the high dose. No difference in cellularity and inflammation in the lungs was apparent between the control and treated groups (data not shown), indicating that RIT had no appreciable effect on the magnitude of the inflammatory response.
Discussion
The mAb 18B7 binds to the capsule of CN and has no inherent fungicidal activity. Radiolabeling mAb 18B7 with either 188Re or 213Bi converts this antibody into a fungicidal molecule in vitro and therapeutic in vivo, as demonstrated by reduced fungal burden and significant prolongation of survival of treated infected mice. No prolongation in survival was observed in mice treated with unlabeled mAb 18B7 relative to the mice given no antibody treatment (PBS) group (P > 0.05). There are two explanations for the lack of efficacy of nonradioactive antibody. First, only a small amount of Ig (30–50 μg) was given per mouse. Second, antibody was administered 24 h after infection. The efficacy of nonradioactive antibody drops dramatically when given after infection in this model, a finding that is commonly observed when therapeutic antibodies are tested in mouse models of acute lethal infection (36).
The reduction in organ cfu observed for mice given RIT indicates that the prolongation of survival is likely a result of reduced organ fungal burden as a consequence of direct microbicidal activity by radiolabeled antibody in vivo. The RIT protocol used in our experiments is theoretically capable of delivering a radioactive atom to every CN cell in this mouse model. At 24 h after i.v. infection with 105 CN cells, there can be expected to be a maximum of 4 × 108 CN yeast cells in the host, assuming a 2-h doubling time with no cell killing by host defenses. Doses of 50, 100, and 200 μCi of 213Bi-CHXA″-18B7 contain 7.5 × 109, 1.5 × 1010, and 3 × 1010 213Bi atoms, respectively; and 50, 100, and 200 μCi of 213Bi-CHXA″-18B7 contain 1.6 × 1011, 3.2 × 1011, and 6.4 × 1011 188Re atoms, respectively. It is noteworthy that during in vitro incubation with radiolabeled antibody, a very high percentage of β-radiation emitted by 188Re is deposited outside of the cell, because 188Re β-particles have a range in tissue of ≈5 mm, and the radius of the CN capsule is only 20 ± 6 μm (37). In vivo, on the other hand, as the density of the microorganisms in infectious foci increases, the absorbed dose from 188Re will increase exponentially. Also, even if a particular cell has not been targeted with a radiolabeled antibody molecule, it may be killed by radiation from a distant cell by the so-called “crossfire” effect. Nevertheless, even under suboptimal in vitro conditions, the antibody-specific effective treatment of CN cells in suspension with 188Re-18B7 was observed. Also, there could be some synergy between the effect of the radioactivity and the antibody itself on the microbial cells, because antibody molecules have been recently reported to catalyze ozone formation, which can promote bacterial killing and inflammation (38). The observation that RIT-treated mice had lower cfu in brain tissue indicates the ability of radiolabeled antibody to penetrate this organ despite the blood–brain barrier. This phenomenon may reflect the increased permeability of the blood–brain barrier that has been reported in experimental cryptococcal meningitis (39).
We administered antibody amounts corresponding to a range of activities, from 50 to 200 μCi per mouse, 4-fold (or 300%) increase in dose, to evaluate the “therapeutic window” of RIT for CN infection in A/JCr mice. Increasing the RIT dose by several-fold will often not improve the survival of the host, and can hasten death because of radiation injury. In experimental RIT, the MTA is usually defined as the highest possible activity under the respective conditions that does not result in any mouse deaths, with the next higher dose level resulting in at least 10% of the mice dying from radiation injury (40). Hence, our ability to increase the RIT dose by 300% indicates a large therapeutic window. By comparison, the MTA for cancer therapy is usually determined by increasing the activities administered in 15–20% increments (41). Presumably, our large therapeutic window reflects the radiolabeled antibody localization to infected sites and sparring of vital organs.
One theoretical impediment to using RIT for the treatment of established cryptococcosis is that patients with this disease often have large amounts of circulating CPS (cryptococcal antigen) that could interfere with RIT by binding radiolabeled mAb. However, we found that the radiolabeled antibody was therapeutic even in mice with serum antigen levels comparable to those found in patients. The most likely explanation for this effect is that the antibody has higher affinity to capsule and tissue polysaccharide than to soluble polysaccharide. This result suggests that RIT can be effective in human cryptococcosis. Incidentally, this concern does not apply to the application of RIT to other infectious diseases where serum antigenemia does not occur.
RIT has been exploited in oncology, because radiolabeled antibodies provide a valuable alternative to chemotherapy and external radiation beam by selectively delivering lethal doses of radiation to cancerous cells (42). The use of RIT for tumor treatment has been studied for several decades, and there is a wealth of knowledge regarding the interaction of radiolabeled antibodies and fragments, both murine and humanized, with target cancer cells, surrounding tissue, and major organs derived from both laboratory and clinical studies (43–45). Certain features of infection make it more amenable for treatment with RIT than malignancies. Solid tumors present challenges such as slow penetration rate of antibodies into tumor tissue and hypoxia, which decreased the efficiency of treatment with β-emitting radioisotopes. In contrast, pathogen-specific antibodies are likely to reach the infectious foci quickly, given that increased capillary permeability at the sites of infection allows radiopharmaceuticals to easily migrate from the circulation (46). We envisage that this method can be applied to obtain dosimetry information before RIT by imaging patients with the antibody labeled with a diagnostic isotope such as 99mTc or 111In. In most infectious diseases, it is not necessary for the treatment to kill every microbe to achieve a therapeutic benefit, because the immune system may effectively control infection when the inoculum is reduced by microbicidal radiation. Longer-lived isotopes such as yttrium-90 (t1/2 = 2.7 d), lutetium-117 (t1/2 = 6.7 d), or Iodine-131 (t1/2 = 8 d) can be used for treatment of infection within abscesses or in the difficult-to-access sites deep in the body. Another advantage of RIT relative to naked antibody therapy is that the latter can be complicated by the occurrence of prozone-like effects at high-antibody concentrations (47). Because the killing power of RIT is based on radiation, and the amounts of antibody administered are low, prozone-like phenomena are not expected to occur.
The data accumulated in clinical RIT of cancer indicate that the primary toxicity of high-dose RIT of infection is likely to be bone marrow suppression. However, when RIT treatment is administered in the doses below the MTA, only transient or no hematological toxicity is observed. In our experiments, we have observed no hematological toxicity for 100 μCi of 188Re-18B7 mAb-treated group of mice, most of whom survived. The highest dose of 200 μCi of 188Re-18B7 mAb was the most efficient in reducing the fungal burden in the lungs of infected mice, but it also caused significant changes in platelet counts, which attest to possible radiotoxicity of this high dose. However, we also note a beneficial hematological effect of radiolabeled antibody on platelet counts that may reflect treatment of the underlying infection. Furthermore, we note recovery of platelet counts in 100 μCi of 188Re-18B7-treated mice, indicating that the toxicity is transient. Examination of lung inflammatory response by histological analysis revealed no appreciable differences. Given the short trajectory of the particle, it is likely that most hits would occur on fungal cells or immune cells in the immediate vicinity. Presumably, as fungal cells are killed, other immune effector cells that are recruited will be more effective in clearing up the site. Important determinants of the extent and duration of myelosuppression include bone marrow reserve (based on prior cytotoxic therapy and extent of disease involvement), total tumor (infection) burden, spleen size, and radioimmunoconjugate stability (43). Clearly, the application of this technology to human cryptococcosis or other infectious diseases will require optimization of the dose to minimize toxicity and additional clinical development. However, we are encouraged that these initial studies in mice suggest that RIT of infection is relatively well tolerated, and may have a significantly higher therapeutic index than RIT of cancer.
The field of infectious diseases is currently facing significant challenges, given an increasing prevalence of highly resistant microorganisms, the emergence of new pathogens, and the existence of large numbers of immunosuppressed individuals in whom standard antimicrobial therapy is often not very effective (48). Furthermore, the specter of biological warfare has raised a new threat to humanity from infectious diseases. Our results provide a proof of principle that RIT can be used as an antiinfective modality and suggest that this approach could be developed for the treatment of various infectious diseases. RIT may be of particular value alone or in combination with standard therapy for the treatment of infections: (i) in special populations such as immunosuppressed patients infected with CN or with other AIDS-associated opportunistic infections; (ii) because of highly resistant microorganisms for which therapeutic options are currently very limited; and (iii) for diseases caused by microbes for which there is no effective antimicrobial chemotherapy. We believe this method can be adapted for use against a variety of difficult to treat infectious diseases, such as Aspergillosis in organ transplant patients. One attraction of developing RIT for infectious diseases is that it represents the “marriage” of two mature technologies, mAbs and nuclear medicine, in an application where the limitations encountered in using this approach for tumor therapy are much less confining.
Supplementary Material
Acknowledgments
We thank Dr. M. Brechbiel (National Institutes of Health) for providing CHXA″ ligand; Drs. C. Taborda, J. Rivera, and Mr. A. Mednick (Albert Einstein College of Medicine) for performing i.v. infection of mice and for help with blood counts; Miss A. Frenkel for help with cfu counts; Dr. L. S. Zuckier (Albert Einstein College of Medicine) for assistance with initial biodistribution; Dr. S. Heller for help with the imaging; and Drs. J. D. Nosanchuk, L.-A. Pirofski, M. D. Blaufox, and M. D. Scharff (Albert Einstein College of Medicine) for critical reviews of the manuscript. The work was supported by Albert Einstein College of Medicine Grant 9526-9593 (to E.D.) and National Institutes of Health Grants AI33774 and AI13342 (to A.C.), AI52042 (to E.D.), and HL59842 (to A.C.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CN, Cryptococcus neoformans; RIT, radioimmunotherapy; CHXA″, N-[2-amino-3-(p-isothiocyanatophenyl)propy1]-trans-cyclohexane-1,2-diamine-N,N′,N″,N′, N″″-pentaacetic acid; CPS, capsular polysaccharide; cfu, colony-forming units.
References
- 1.Casarett, A. P. (1968). Radiation Biology (Prentice–Hall, Englewood Cliffs, NJ).
- 2.Dembitzer, H. M., Buza, I. & Reiss, F. (1972) Mycopathol. Mycol. Appl. 47, 307–315. [DOI] [PubMed] [Google Scholar]
- 3.Zack, M. B., Stottemier, K., Berg, G. & Kazemi, H. (1974) Chest 66, 240–243. [DOI] [PubMed] [Google Scholar]
- 4.Dadachova, E. (1999) J. Labelled Comp. Radiopharm. 42, 287–292. [Google Scholar]
- 5.Goldenberg, D. M., Sharkey, R. M., Udem, S., Vagg, R., Levine, G. M., Conte, P., Swayne, L. C., Hansen, H. J., Cunniff, D., Anton, J., et al. (1994) J. Nucl. Med. 35, 1028–1034. [PubMed] [Google Scholar]
- 6.Malpani, B. L., Kadival, G. V. & Samuel, A. M. (1992) Nucl. Med. Biol. 19, 45–53. [DOI] [PubMed] [Google Scholar]
- 7.Becker, W., Bair, J., Behr, T., Repp, R., Streckenbach, H., Beck, H., Gramatzki, M., Winship, M. J., Goldenberg, D. M. & Wolf, F. (1994) J. Nucl. Med. 35, 1436–1443. [PubMed] [Google Scholar]
- 8.Casadevall, A. & Pirofski, L. (2001) Clin. Infect. Dis. 33, 1048–1056. [DOI] [PubMed] [Google Scholar]
- 9.Spitzer, E. D., Spitzer, S. G., Freundlich, L. F. & Casadevall, A. (1993) Lancet 341, 595–596. [DOI] [PubMed] [Google Scholar]
- 10.Currie, B. P. & Casadevall, A. (1994) Clin. Infect. Dis. 19, 1029–1033. [DOI] [PubMed] [Google Scholar]
- 11.Lendvai, N. & Casadevall, A. (1999) J. Infect. Dis. 180, 791–801. [DOI] [PubMed] [Google Scholar]
- 12.Knapp, F. F., Jr. (1998) Cancer Biother. Radiopharm. 13, 337–349. [DOI] [PubMed] [Google Scholar]
- 13.Hoher, M., Wohrle, J., Wohlfrom, M., Hanke, H., Voisard, R., Osterhues, H. H., Kochs, M., Reske, S. N., Hombach, V., Kotzerke, J. (2000) Circulation 101, 2355–2360. [DOI] [PubMed] [Google Scholar]
- 14.Palmedo, H., Guhlke, S., Bender, H., Sartor, J., Schoeneich, G., Risse, J., Grunwald, F., Knapp, F. F. Jr., & Biersack, H. J. (2000) Eur. J. Nucl. Med. 27, 123–130. [DOI] [PubMed] [Google Scholar]
- 15.McDevitt, M. R., Barendswaard, E., Ma, D., Lai, L., Curcio, M. J., Sgouros, G., Ballangrud, A. M., Yang, W. H., Finn, R. D., Pellegrini, V., et al. (2000) Cancer Res. 60, 6095–6100. [PubMed] [Google Scholar]
- 16.Behr, T. M., Behe, M., Stabin, M. G., Wehrmann, E., Apostolidis, C., Molinet, R., Strutz, F., Fayyazi, A., Wieland, E., Gratz, S., et al. (1999) Cancer Res. 59, 2635–2643. [PubMed] [Google Scholar]
- 17.Kennel, S. J. & Mirzadeh, S. (1998) Nucl. Med. Biol. 25, 241–246. [DOI] [PubMed] [Google Scholar]
- 18.Adams, G. P., Shaller, C. C., Chappell, L. L., Wu, C., Horak, E. M., Simmons, H. H., Litwin, S., Marks, J. D., Weiner, L. M. & Brechbiel, M. W. (2000) Nucl. Med. Biol. 27, 339–346. [DOI] [PubMed] [Google Scholar]
- 19.Kolbert, K. S., Hamacher, K. A., Jurcic, J. G., Scheinberg, D. A., Larson, S. M. & Sgouros, G. (2001) J. Nucl. Med. 42, 27–32. [PubMed] [Google Scholar]
- 20.Sgouros, G., Ballangrud, A. M., Jurcic, J. G., McDevitt, M. R., Humm, J. L., Erdi, Y. E., Mehta, B. M., Finn, R. D., Larson, S. M. & Scheinberg, D. A. (1999) J. Nucl. Med. 40, 1935–1946. [PubMed] [Google Scholar]
- 21.Casadevall, A., Mukherjee, J., Devi, S. J., Schneerson, R., Robbins, J. B. & Scharff, M. D. (1992) J. Infect. Dis. 165, 1086–1093. [DOI] [PubMed] [Google Scholar]
- 22.Casadevall, A., Mukherjee, J. & Scharff, M. D. (1992) J. Immunol. Methods 154, 27–35. [DOI] [PubMed] [Google Scholar]
- 23.Dadachova, E., Mirzadeh, S., Smith, S. V., Knapp, F. F. & Hetherington, E. L. (1997) Appl. Radiat. Isot. 48, 477–481. [DOI] [PubMed] [Google Scholar]
- 24.Pippin, C. G., Parker, T. A., McMurry, T. J. & Brechbiel, M. W. (1992) Bioconjugate Chem. 3, 342–345. [DOI] [PubMed] [Google Scholar]
- 25.Boll, R. A., Mirzadeh, S. & Kennel, S. J. (1997) Radiochim. Acta 79, 145–149. [Google Scholar]
- 26.Early, P. J. & Sodee, D. B. (1995) in Principles and Practice of Nuclear Medicine (Mosby, St. Louis), pp. 118–120.
- 27.Dadachova, E. & Chapman, J. (1998) Nucl. Med. Commun. 19, 173–181. [PubMed] [Google Scholar]
- 28.Senekowitsch-Schmidtke, R., Schuhmacher, C., Becker, K. F., Nikula, T. K., Seidl, C., Becker, I., Miederer, M., Apostolidis, C., Adam, C., Huber, R., et al. (2001) Cancer Res. 61, 2804–2808. [PubMed] [Google Scholar]
- 29.Dadachova, E. & Mirzadeh, S. (1997) Nucl. Med. Biol. 24, 605–608. [DOI] [PubMed] [Google Scholar]
- 30.Mirzadeh, S., Brechbiel, M. W., Atcher, R. W. & Gansow, O. A. (1990) Bioconjugate Chem. 1, 59–65. [DOI] [PubMed] [Google Scholar]
- 31.Feldmesser, M. & Casadevall, A. (1997) J. Immunol. 158, 790–799. [PubMed] [Google Scholar]
- 32.Rhodes, J. C., Wicker, L. S. & Urba, W. (1980) Infect. Immun. 29, 494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukherjee, J., Zuckier, L. S., Scharff, M. D. & Casadevall, A. (1994) Antimicrob. Agents Chemother. 38, 580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miale, J. B. (1982) Laboratory Medicine Hematology (Mosby, St. Louis).
- 35.Wong, J. Y., Thomas, G. E., Yamauchi, D., Williams, L. E., Odom-Maryon, T. L., Liu, A., Esteban, J. M., Neumaier, M., Dresse, S., Wu, A. M., et al. (1997) J. Nucl. Med. 38, 1951–1959. [PubMed] [Google Scholar]
- 36.Casadevall, A. & Scharff, M. D. (1994) Antimicrob. Agents Chemother. 38, 1695–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rivera, J., Feldmesser, M., Cammer, M. & Casadevall, A. (1998) Infect. Immun. 66, 5027–5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wentworth, P., Jr., McDunn, J. E., Wentworth, A. D., Takeuchi, C., Nieva, J., Jones, T., Bautista, C., Ruedi, J. M., Gutierrez, A., Janda, K. D., et al. (2002) Science 298, 2195–2199. [DOI] [PubMed] [Google Scholar]
- 39.Goldman, D. L., Casadevall, A., Cho, Y. & Lee, S. C. (1996) Lab. Invest. 75, 759–770. [PubMed] [Google Scholar]
- 40.Behr, T. M., Behe, M., Lohr, M., Sgouros, G., Angerstein, C., Wehrmann, E., Nebendahl, K. & Becker, W. (2000) Eur. J. Nucl. Med. 27, 753–765. [DOI] [PubMed] [Google Scholar]
- 41.Sharkey, R. M., Blumenthal, R. D., Behr, T. M., Wong, G. Y., Haywood, L., Forman, D., Griffiths, G. L. & Goldenberg, D. M. (1997) Int. J. Cancer 72, 477–485. [DOI] [PubMed] [Google Scholar]
- 42.Goldenberg, D. M. (2002) J. Nucl. Med. 43, 693–699. [PubMed] [Google Scholar]
- 43.Knox, S. J. & Meredith, R. F. (2000) Semin. Radiat. Oncol. 10, 73–93. [DOI] [PubMed] [Google Scholar]
- 44.Milenic, D. E. (2000) Semin. Radiat. Oncol. 10, 139–155. [DOI] [PubMed] [Google Scholar]
- 45.Buchsbaum, D. J. (2000) Semin. Radiat. Oncol. 10, 156–167. [DOI] [PubMed] [Google Scholar]
- 46.Becker, W. (1995) Eur. J. Nucl. Med. 22, 1195–1211. [DOI] [PubMed] [Google Scholar]
- 47.Taborda, C. P. & Casadevall, A. (2001) J. Immunol. 166, 2100–2107. [DOI] [PubMed] [Google Scholar]
- 48.Casadevall, A. (1996) Clin. Infect. Dis. 23, 790–794. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.