Abstract
Originally ascribed passive roles in the CNS, astrocytes are now known to have an active role in the regulation of synaptic transmission. Neuronal activity can evoke Ca2+ transients in astrocytes, and Ca2+ transients in astrocytes can evoke changes in neuronal activity. The excitatory neurotransmitter glutamate has been shown to mediate such bidirectional communication between astrocytes and neurons. We demonstrate here that ATP, a primary mediator of intercellular Ca2+ signaling among astrocytes, also mediates intercellular signaling between astrocytes and neurons in hippocampal cultures. Mechanical stimulation of astrocytes evoked Ca2+ waves mediated by the release of ATP and the activation of P2 receptors. Mechanically evoked Ca2+ waves led to decreased excitatory glutamatergic synaptic transmission in an ATP-dependent manner. Exogenous application of ATP does not affect postsynaptic glutamatergic responses but decreased presynaptic exocytotic events. Finally, we show that astrocytes exhibit spontaneous Ca2+ waves mediated by extracellular ATP and that inhibition of these Ca2+ responses enhanced excitatory glutamatergic transmission. We therefore conclude that ATP released from astrocytes exerts tonic and activity-dependent down-regulation of synaptic transmission via presynaptic mechanisms.
Astrocytes are the predominant glial cell type in the CNS and are intimately associated with neurons. Originally thought to being purely supportive in the CNS, astrocytes are now known to have active roles in the modulation of neuronal activity and synaptic neurotransmission (1). Astrocytes lack the ability to propagate regenerative electrical signals but are nonetheless responsive to a variety of extracellular stimuli and produce regenerative Ca2+ waves that spread within astrocyte networks (2–4). Ca2+ signals in astrocytes can evoke the release of neuroactive substances, such as glutamate and ATP, which can lead to activation of neuronal receptors and increases in neuronal Ca2+ levels (5).
The first evidence for dynamic communication from astrocytes to neurons came from the discovery of temporally related changes in intracellular Ca2+ concentration ([Ca2+]i) in glial and neuronal cells. Various stimuli that selectively elevate [Ca2+]i in astrocytes lead to delayed elevations in [Ca2+]i in neurons in culture (6). In hippocampal slice preparations, activation of metabotropic glutamate receptors in astrocytes evokes Ca2+ signals in astrocytes which are followed by a delayed elevation of neuronal Ca2+ levels (7, 8). Evidence suggests that such Ca2+-mediated extracellular signaling between astrocytes and neurons may be implicated in the regulation of synaptic transmission. Stimulation of Ca2+ waves in astrocytes can increase both excitatory and inhibitory postsynaptic currents in hippocampal cultures (9). In the retina, astrocytic Ca2+ waves can modulate light-induced excitation of ganglion cells (10). Glutamate appears to be an important mediator for these astrocyte-to-neuron signals.
There is an increasing body of evidence, however, showing that ATP, the predominant extracellular signaling molecule among astrocytes (3, 11–13), may also mediate signaling between neurons and glial cells (14). Neurons are known to express a wide variety of ionotropic (P2X) and metabotropic (P2Y) receptor subtypes in the pre- and postsynaptic regions. Given that astrocytic Ca2+ waves can evoke changes in neuronal synaptic activity and that Ca2+ waves are mediated by the release of ATP, ATP released from astrocytes may be involved in astrocyte-to-neuron signaling in synaptic regions of the CNS.
In this study, we investigated the effects of Ca2+ wave stimulation in astrocytes on the synaptic activity of neurons in hippocampal cultures. We demonstrate that the release of ATP from astrocytes after stimulation of Ca2+ waves evokes a decrease in the glutamatergic synaptic transmission. We also demonstrate that such actions of astrocytes occur even in a tonic fashion.
Materials and Methods
Culture of Hippocampal Astrocytes and Neurons. All of the animals used in the present study have been obtained, housed, cared for, and used in accordance with the guidelines of National Institute of Health Sciences. Cocultured hippocampal neurons and glial cells were prepared as described (15). The same method was applied for culturing hippocampal astrocytes, except that the hippocampal cortices were dissected from newborn Wistar rats (16). To purify astrocytes from hippocampal cultures, the cells were subjected to 24 h of continuous shaking 3–4 days after plating, and detached cells were removed. Over 93% of such cells were positive to anti-glial fibrillary acidic protein (GFAP).
Ca2+ Imaging in Single Hippocampal Cells. Changes in [Ca2+]i in single cells were measured by the fura 2 method with minor modifications (17). In brief, the culture medium was replaced with balanced salt solution (BSS) of the following composition (in mM): NaCl 150, KCl 5.0, CaCl2 1.8, MgCl2 1.2, N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (Hepes) 25, and d-glucose 10 (pH 7.4). Cells were loaded with fura 2 by incubation with 5 μM fura 2-acetoxymethyl ester (fura 2-AM, Molecular Probes) at room temperature in BSS for 45 min. The coverslips were mounted on an inverted epifluorescence microscope (TMD-300, Nikon) equipped with a 75-W xenon lamp and band-pass filters of 340 and 360 nm wavelengths. Image data were recorded by a silicon intensifier target camera (C-2741-08, Hamamatsu Photonics, Hamamatsu City, Japan). For mechanical stimulation, a single astrocyte in the microscopic field was probed with a microglass pipette by using a micromanipulator (Narishige, Tokyo). Under visible light, the tip of the micropipette was positioned ≈2 μm over the cell to be stimulated. The micropipette was rapidly lowered ≈2 μm and then rapidly returned to its original position. If the stimulated cell showed any sign of damage (dye leakage or abnormal morphology), the experiment was eliminated. For confocal Ca2+ imaging, the cells were loaded with 5 μM fluo-4AM for 30–40 min at room temperature and mounted on a microscope (E-600 or TE-2000, Nikon) equipped with a CSU-10 laser-scanning unit (Yokogawa, Tokyo) and charge-coupled device (CCD) camera (ORCA-ER, Hamamatsu Photonics) as described (18, 19).
Imaging of ATP Release. The cell chamber was filled with a luciferin–luciferase reagent (ATP bioluminescence assay kit CLS II, Roche Diagnostics), and then release of ATP in response to mechanical stimulation was visualized as described by Wang et al. (20), with minor modifications. ATP bioluminescence was detected with a high-sensitivity CCD camera (C6790-80, Hamamatsu Photonics) with an image intensifier (C8600-03, Hamamatsu Photonics) in a dark box. Images of ATP release were collected at 1-s intervals with exposure times of 500 ms. The absolute ATP concentration was estimated by using standard ATP solution (0.01–1.0 μM).
Glutamate Release. The amount of glutamate release was determined by HPLC-ECD (ECD-300, Eicom, Kyoto) with an enzymatic column (E-ENZ, Eicom) containing glutamate oxidase that reacts with glutamate to generate H2O2 (21). Cells were stimulated with high K+ (50 mM) in the presence and absence of various concentration of ATP for 1 min. In this study, we used Krebs–Ringer bicarbonate solution instead of BSS to exclude an interfering peak induced by Hepes.
Optical Analysis of Secretory Responses in Single Cells. A slightly modified FM1-43 method (22) was used for monitoring of presynaptic functions. In brief, cells are stained by exposure to FM1-43 (10 μM) in high K+ (50 mM KCl) for 1 min, which was followed by a wash and a further 20 min-incubation to allow endocytosis in the presence of FM1-43. Cells were then washed by dye-free BSS for 30 min to remove nonspecific binding of FM1-43. Images were obtained with an inverted microscope (TE-2000, Nikon) equipped with a CCD camera (ORCA-ER, Hamamatsu Photonics).
Immunocytochemical Study. After fixation, cells were incubated with primary antibodies (rabbit anti-GFAP antibody 1:1,000; mouse anti-MAP2a and b monoclonal antibody 1:200; Chemicon) dissolved in blocking solution (Blockace, Dainihon, Osaka) for 24 h at 4°C. Then cells were visualized by Alexa488- and Alexa546-conjugated rabbit- and mouse-anti-IgGs, respectively. Images were obtained by confocal microscopy (Radiance 2000, Bio-Rad).
Source of Reagents. All other reagents, except where indicated above, were from Sigma.
Results
Ca2+ Waves in Hippocampal Astrocytes Require Extracellular ATP and P2 Receptors. Mechanical stimulation of a single cultured hippocampal astrocyte (Fig. 1a, cell 1) typically evoked Ca2+ waves in which an elevation in [Ca2+]i evoked in the stimulated cell was followed by an increase in [Ca2+]i in the neighboring astrocytes (Fig. 1 a and c). Mechanically evoked Ca2+ waves spread concentrically at an average velocity of 21 ± 1.7 μm/s to an average radius (φCa2+) of 206 ± 16 μm(n = 37). In the presence of bath-applied apyrase (grade III; 20 units/ml), an ATP-degrading enzyme, a [Ca2+]i rise in the stimulated cell was followed by increases in [Ca2+]i in only one of the three the neighboring cells, indicating attenuation of the Ca2+ wave (Fig. 1 b and d). On average, the spread of the Ca2+ waves was reduced by 75.4 ± 8.2% (n = 34) in the presence of apyrase. As summarized in Table 1, similar attenuation of mechanically evoked Ca2+ waves was observed in the presence of the P2 ATP receptor antagonists suramin (100 μM) and PPADS (30 μM). On the other hand, the spread of Ca2+ waves was not significantly attenuated in the presence of the gap junction blocker octanol (500 μM), nor in the presence of (±)-α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid hydrate (AMPA) receptor antagonist CNQX (100 μM). The metabotropic glutamate receptor antagonist MCPG (100 μM) slightly attenuated the spread of Ca2+ wave propagation (Table 1). These results demonstrate that mechanically evoked Ca2+ waves in hippocampal astrocytes are mediated by the release of ATP and the activation of P2 receptors.
Fig. 1.
Mechanically evoked Ca2+ waves in astrocytes are attenuated by apyrase. (a) Phase-contrast image (leftmost panel) and ratiometric images of fura 2 fluorescence images of a field of hippocampal cultured cells in which an astrocyte (cell 1) was mechanically stimulated by gentle contact with a glass pipette. Ratiometric images were taken during stimulation (0 s) and 4 and 12 s after stimulation. (b) Phase-contrast image (leftmost panel) and ratiometric images of fura 2 fluorescence in a different field of hippocampal astrocytes in which cell 1 was mechanically stimulated in the presence of apyrase (grade III, 20 units/ml). (Scale bar in a and b, 100 μm.) (c) The graph shows plots of ratiometric fura 2 fluorescence as a function of time in the four individual astrocytes indicated in the leftmost panel in a. The plots for the nonstimulated cells (cells 2–4) are displaced horizontally in proportional to their distance from the stimulated cell (cell 1) as indicated by the scale. (Inset) The traces from the stimulated cell (dark trace) and neighboring cells (light traces) are temporally aligned for the time course indicated by the hatched bar in the graph. The arrowhead indicates mechanical stimulation. (d) Same as c for the cells shown in the leftmost panel in b in which cell 1 was mechanically stimulated in the presence of apyrase.
Table 1. Pharmacological characterization of propagating Ca2+ waves in response to mechanical stimulation in astrocytes.
| Reagents | Radius, μm (n) |
|---|---|
| Control | 206 ± 16 (37) |
| Apyrase (20 units/ml) | 56 ± 8 (34) |
| Suramin (100 μM) | 30 ± 6 (6) |
| PPADS (30 μM) | 33 ± 4 (4) |
| Octanol (500 μM) | 217 ± 21 (8) |
| CNQX (100 μM) | 187 ± 21 (8) |
| MCPG (100 μM) | 158 ± 23 (11) |
Data show mean ± SEM of radius of Ca2+ waves in the presence of various reagents. CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; MCPG, (S)-α-methyl-(4-carboxyphenyl)glycine; PPADS, 4-[[4-formyl-5-hydroxy-6-methyl-3-[(phosphonooxy)methyl]-2-pyridinyl]azo]-1,3-benzenedisulfonic acid.
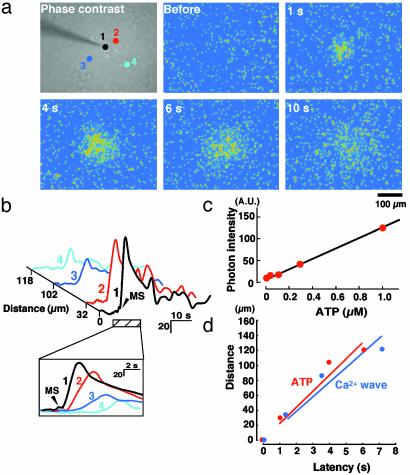
Initiation of Ca2+ Waves in Astrocytes Causes Increases in Extracellular ATP. To directly demonstrate the stimulus-evoked release of ATP from astrocytes, we modified the luciferin–luciferase chemiluminescence bioassay by using a high sensitivity CCD camera coupled with an image intensifier to correlate ATP-mediated bioluminescence with increases in extracellular ATP concentration ([ATP]). As shown in Fig. 2c, a standard calibration curve obtained from this system shows a high correlation between chemiluminescence intensity and [ATP], with correlation coefficient of 0.99 over an [ATP] range of 0.01–1 μM. Hippocampal cultures were bathed in solution containing the luciferin–luciferase reagents, and the chemiluminescence intensity was collected at 1-s intervals with exposure times of 500 ms. A typical temporal and spatial pattern of extracellular [ATP] changes after mechanical stimulation of an astrocyte is shown in Fig. 2a. From the calibration curve (Fig. 2c), we estimated the maximal [ATP] at the stimulated site to be 340.5 ± 38.2 nM (n = 9), a concentration that is sufficient to activate P2 receptors and evoke [Ca2+]i increases in astrocytes (16). Fig. 2d shows the velocity of release of ATP (red) in a typical experiment, comparing with that of the Ca2+ wave (blue). The ATP signals strongly associated with evoked Ca2+ waves, and the mean velocity (within 150 μm apart from the stimulated site) for ATP signals and Ca2+ waves was 22.5 ± 2.2 μm/s (n = 9) and 21 ± 1.7 μm/s(n = 37), respectively. Therefore, waves of ATP release are temporally correlated with Ca2+ waves in astrocytes, thus providing further support that Ca2+ waves in astrocytes are mediated by the release of ATP.
Fig. 2.
Mechanical stimulation of astrocytes evokes an increase in extracellular ATP. (a) A phase-contrast image (Upper Left) and pseudocolor images of ATP bioluminescence in astrocytes. Astrocytes were bathed in luciferin–luciferase reagent and stimulated mechanically. ATP images were taken by a high-sensitivity CCD camera coupled with an image intensifier at an exposure time of 500 ms. (b) The graph shows plots of ATP bioluminescence as a function of time in the four individual astrocytes indicated in the phase-contrast image in a. The plots for the nonstimulated cells (cells 2–4) are displaced horizontally in proportion to their distance from the stimulated cell (cell 1), as indicated by the scale. (Inset) Traces from the stimulated cell (dark trace) and neighboring cells (traces 2–4) are temporally aligned for the time course indicated by the hatched bar in the graph. The standard calibration curve obtained from our imaging method is shown in c. Each ATP standard solution was injected into the chamber, and then the averaged ATP chemiluminescence of the whole microscopic field was obtained with exposure times of 500 ms. The correlation coefficient was 0.99. (d) Comparison of the spread of ATP signals and Ca2+ waves in typical coverslips. Four different astrocytes were chosen in the microscopic field, and each distance from the stimulated cell was plotted against its latency for ATP signals (red) and Ca2+ waves (blue). ATP signals and Ca2+ waves were obtained from different coverslips. The averaged velocity for ATP signals and Ca2+ waves was 22.5 ± 2.2 μm/s(n = 9) and 21 ± 1.7 μm/s(n = 37), respectively.
ATP-Dependent Astrocytic Ca2+ Wave Appears to Be a Trigger for Decreasing Neuronal Ca2+ Oscillation. We have previously shown that cultured hippocampal neurons exhibit synchronized Ca2+ oscillations (17). These neuronal Ca2+ oscillations are mediated by glutamatergic excitatory synaptic transmission because they are abolished by tetrodotoxin (TTX), 0 Ca2+, or antagonists to ionotropic glutamate receptors (17). We therefore used such neuronal Ca2+ oscillations as an index of excitatory synaptic transmission to investigate the effect of mechanically evoked Ca2+ waves in astrocytes on neuronal activity. Immunostaining of hippocampal cultures with the neuronal marker anti-MAP2 and the astrocyte marker anti-glial fibrillary acidic protein (GFAP) after Ca2+ imaging experiments confirmed our ability to visually identify neurons and astrocytes (Fig. 3a). Before mechanical stimulation of an astrocyte, the frequency of neuronal Ca2+ oscillations was 5.7 ± 0.7 per min (Fig. 3 c, cells 4–6, and d, traces 4–6). Mechanical stimulation of an astrocyte (Fig. 3c, cell 1) evoked an increase in [Ca2+]i in the stimulated cell that was followed by transient rises in [Ca2+]i in neighboring astrocytes (Fig. 3 c and d, cells 2 and 3) and ≈30% of the neurons (23/73). Stimulation of a Ca2+ waves also caused a significant decrease in the frequency of synchronized neuronal Ca2+ oscillations in almost all neighboring neurons regardless of whether they exhibited the transient [Ca2+]i elevations in response to mechanical stimulation of the astrocytes. Among the neurons shown in Fig. 3c, stimulation of the astrocyte caused a 78.5 ± 6.7% decrease in the frequency of synchronized Ca2+ oscillations within 1–2 min that gradually returned to prestimulation levels within 4 min after the mechanical stimulation (Fig. 3e). The mean distance of these neurons from the stimulated cells was 105.6 ± 10.2 μm(n = 73), a distance that is within the typical range of the Ca2+ waves spread in astrocytes (Table 1). When the astrocyte was stimulated twice (MS1 and MS2 in Fig. 3d), the second stimulation (MS2) evoked similar Ca2+ waves in astrocytes, which also caused a decrease in the frequency the neuronal Ca2+ oscillations (Fig. 3 d, traces 4 and 5, and e). Similar decreases in neuronal Ca2+ oscillations were observed after the application of exogenous ATP (1 μM; Fig. 3 b and h). In the presence of apyrase, however, mechanical stimulation of astrocytes failed to evoke neuronal Ca2+ transients or significant decreases in the frequency of synchronized neuronal Ca2+ oscillations (Fig. 3 f–h). These results suggest that transient neuronal Ca2+ responses and decreases in the frequency of neuronal Ca2+ oscillations observed after the stimulation of Ca2+ waves appear to be mediated by extracellular ATP.
Fig. 3.
Stimulation of astrocytic Ca2+ waves causes a decrease in the frequency of neuronal Ca2+ oscillations. (a) Image of a field of hippocampal cultures that were fixed and stained with anti-MAP2 (green) and anti-GFAP (red) antibodies. (Scale bar, 50 μm.) (b) The graph shows individual traces of ratiometric fura 2 fluorescence in a neuron (cell 1) and astrocyte (cell 2) shown in a before fixation and staining. ATP (1 μM) was bath applied as indicated by the bar. (c Left) Ratiometric image of fura 2 fluorescence superimposed on a phase-contrast image of a field of hippocampal cultured cells. (c Right) Astrocytes (cells 1–3in Left) are schematically shown as red and neurons (cells 4–6) are shown as blue. Astrocyte 1 was mechanically stimulated as indicated by the arrowhead. (Scale bar, 50 μm.) (d) The graph shows individual traces of ratiometric fura 2 fluorescence as a function of time in the astrocytes (traces 1–3) and neurons (4–6) indicated in c. Astrocyte 1 was mechanically stimulated twice, separated by 10 min (the arrowheads, MS1 and MS2). (e) The histogram shows the mean ± SEM frequency of Ca2+ oscillations in neurons (n = 73 neurons in four experiments) measured every 60 s before and after an astrocyte was mechanically stimulated (arrowheads). The Ca2+ oscillation frequency was normalized to the prestimulation level. (f) Ratiometric and schematic images of a field of hippocampal cultured cells in which astrocytes are shown in red and neurons are shown in blue. (Scale bar, 50 μm.) (g) Individual traces of ratiometric fura 2 fluorescence in the astrocytes (1–3) and neurons (4–7) shown in f. Astrocyte 1 was mechanically stimulated (arrowheads) under normal conditions and 10 min later was mechanically stimulated in the presence of apyrase (20 units/ml; hatched bar). (h) The histogram shows the mean ± SEM frequency of Ca2+ oscillations in neurons before stimulation (Pre; n = 48 neurons in five experiments), after the application of exogenous ATP (1 μM; n = 36 neurons in five experiments), mechanical stimulation of astrocytes (MS; n = 51 neurons in six experiments), and mechanical stimulation of astrocytes in the presence of apyrase (MS/apyrase; n = 38 neurons in five experiments). Ca2+ oscillation frequency was normalized to the prestimulation level (Pre). Asterisks show significant difference from the response of Pre (**, P < 0.01; Student's t test).
Effects of ATP on Synaptic Transmission Are Presynaptic. We have previously demonstrated that ATP does not affect the postsynaptic glutamatergic responses in hippocampal neurons (17). This suggests that the locus of ATP-dependent decrease in glutamatergic transmission caused by astrocytic Ca2+ waves is a presynaptic change, possibly due to attenuation of exocytotic glutamate release. To determine whether ATP is able to cause such attenuation, we visualized exocytotic events at single hippocampal synapses by using FM1-43 dye (22). FM1-43 signals were strongly colocalized with those of the vesicular protein marker anti-synaptophysin, indicating that FM1-43 signals were localized to nerve terminals (Fig. 4 b–d). On neuronal depolarization with KCl (50 mM), the FM1-43 signals gradually decreased in intensity, indicating exocytotic neuronal release (Fig. 4e). Synaptic neurotransmitter release is highly dependent on extracellular Ca2+. Indeed, the KCl-induced decreases in FM1-43 staining were completely inhibited in the presence of the Ca2+ channel blocker Conotoxin GVIA (ω-Ctx) (Fig. 4e) and after the removal of extracellular Ca2+ (data not shown). The KCl-evoked decreases in FM1-43 fluorescence were also significantly attenuated in the presence of ATP (0.3 to ≈30 μM; Fig. 4 e and f). ATP can therefore attenuate presynaptic exocytotic release. To demonstrate whether ATP inhibits release of glutamate, we directly measured the amount of extracellular glutamate level by HPLC. The amount of glutamate release under basal conditions and after application of high (50 mM) K+-evoked release of glutamate was 6.0 ± 1.3 and 28.1 ± 3.7 pmol per well, respectively. ATP applied at concentrations sufficient to attenuate KCl-evoked decreases in FM1-43 staining (0.3–30 μM) also inhibited the high K+-evoked glutamate release in a concentration-dependent fashion (Fig. 4f Inset).
Fig. 4.
Exogenous ATP causes a decrease in presynaptic exocytotic events. (a) A phase-contrast image of cultured hippocampal neurons. (b) Image of FM1-43 fluorescence in the same field as a. (c) Same field of hippocampal neurons as in a fixed and stained with anti-synaptophysin antibody after KCl (50 mM)-induced depolarization. (d) Merged image of FM1-43 and anti-synaptophysin fluorescence. (e) The graph shows the mean ± SEM levels of relative FM1-43 fluorescence before and after the application of KCl (50 mM) under control conditions (b; n = 46), in the presence of ω-Conotoxin GVIA (ω-CTx; 1 μM; n = 18) and ATP (0.3 μM, n = 41; 3 μM, n = 16; 30 μM, n = 36). (f) The mean ± SEM amount of release 50 s after the application of KCl under control conditions (open bar, n = 46), in the presence of various concentration of ATP (red bars; n = 16∼41) and in the presence of ω-CTx (n = 18). (Inset) Effects of various concentrations of ATP (red bars; 0.1–30 μM, n = 10–15) on the KCl-evoked (50 mM) glutamate release measured by HPLC. Values are normalized to those evoked under control conditions. Asterisks show significant differences from the response of KCl alone (*, P < 0.05; **, P < 0.01; Student's t test).
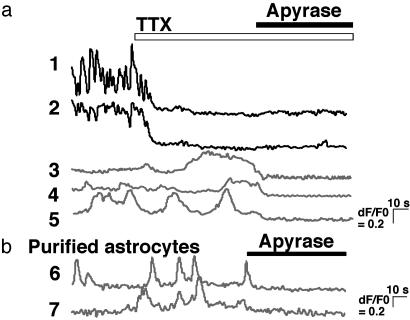
Tonic ATP-Dependent Regulation of Synaptic Transmission. We next monitored the [Ca2+]i in astrocytes and neurons by using confocal laser microscopy. We found that astrocytes (Fig. 5a, traces 3–5) in mixed hippocampal cultures exhibited spontaneous Ca2+ oscillations that were less frequent, smaller in amplitude, and less synchronized than those observed in neurons (Fig. 5a, traces 1 and 2). Unlike neuronal Ca2+ oscillations, those in astrocytes persisted in the presence of TTX applied to the bath (Fig. 5a), suggesting that they do not depend on neuronal activity. Subsequent application of apyrase, however, abolished the spontaneous Ca2+ oscillations in 84 of 98 astrocytes tested (Fig. 5a, traces 3–5). Apyrase-sensitive spontaneous Ca2+ oscillations were also observed in purified hippocampal astrocyte cultures (Fig. 5b, traces 6 and 7). Hippocampal astrocytes therefore exhibit spontaneous Ca2+ oscillations that do not require neuronal activity but do depend on extracellular ATP.
Fig. 5.
Astrocytic Ca2+ oscillations are abolished by apyrase. (a) Self-ratios of fluo-4 fluorescence in cocultured hippocampal neurons (traces 1 and 2) and astrocytes (traces 3–5) obtained from confocal laser microscopy. TTX (1 μM) was applied as indicated by the open bar, and apyrase (20 units/ml) was applied as indicated by the filled bar. (b) Self-ratios of fluo-4 fluorescence in purified astrocyte cultures (traces 6 and 7). Apyrase (20 units/ml) was applied as indicated by the bar.
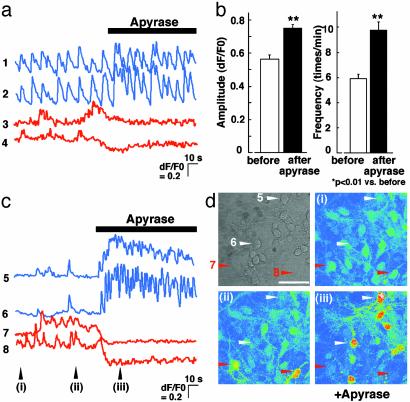
We have demonstrated that the release of ATP is concomitant with the propagation of Ca2+ waves in astrocytes and that Ca2+ waves in astrocytes are able to attenuate the Ca2+ oscillations in neurons in an ATP-dependent manner. Furthermore, astrocytes exhibit spontaneous Ca2+ oscillations that require extracellular ATP, suggesting that there may be tonic increases in the extracellular ATP levels that may tonically regulate the neuronal activity. To determine whether neuronal activity is tonically regulated in an ATP-dependent manner, we investigated the effects of apyrase on the spontaneous synchronized neuronal activity without astrocytic stimulation. We found that apyrase (20 units/ml) significantly enhanced both the amplitude and frequency of the neuronal Ca2+ oscillations (Fig. 6 a, traces 1 and 2, and b). The astrocytic Ca2+ responses, however, were abolished after the application of apyrase (Fig. 6a, traces 3 and 4). Fig. 6d shows a phase-contrast image (Upper Left) and pseudocolor images (i-iii) of fluo-4 self-ratios at time points i, ii, and iii in Fig. 6c. Traces 5–8 in Fig. 6c correspond to cells in 5–8 with arrowheads in the Fig. 6d. In the neurons that exhibited particularly small and infrequent Ca2+ oscillations, application of apyrase was followed by a burst-like increase in both the amplitude and frequency of neuronal Ca2+ oscillations (Fig. 6c, traces 5 and 6), whereas the Ca2+ oscillations in neighboring astrocytes were abolished (traces 7 and 8). Although neuronal activities varied among coverslips, the frequency of neuronal Ca2+ oscillations appeared to be inversely correlated with the initial levels in [Ca2+]i in astrocytes, which depended on extracellular ATP (data not shown). Put together, these results demonstrate that synaptic transmission in hippocampal neurons is tonically down-regulated by astrocytes in an ATP-dependent manner.
Fig. 6.
Frequency and amplitude of neuronal Ca2+ oscillations are increased by apyrase. (a) Self-ratios of fluo-4 fluorescence in cocultured hippocampal neurons (traces 1 and 2) and astrocytes (traces 3 and 4) obtained from confocal laser microscopy. Apyrase (20 units/ml) was applied as indicated by the bar. (b) The mean ± SEM amplitude (Left) and frequency (Right) of neuronal Ca2+ oscillations before (open bar) and after (filled bar) application of apyrase. (c) Self-ratios of fluo-4 fluorescence in a representative example of a field of hippocampal neurons (traces 5 and 6) and astrocytes (traces 7 and 8) in which the neurons exhibited relatively small Ca2+ oscillations. Apyrase (20 units/ml) was applied as indicated by the bar. (d) A phase-contrast image and pseudocolor images (i–iii) of fluo-4 self-ratios at time points i, ii, and iii in c. (Scale bar, 50 μm.)
Discussion
In the present study, we have identified ATP as a signaling molecule released by astrocytes that inhibits synaptic transmission in neurons. This finding of an ATP-mediated signaling system between astrocytes and neurons complements a growing body of evidence which suggests that, in addition to their various supportive roles for neurons, astrocytes are actively involved in the control of synaptic transmission.
Glutamate is the predominant signaling molecule among the previously reported mechanisms through which astrocytes can actively regulate synaptic transmission. In cultured hippocampal neurons, the stimulation of astrocytes evokes a regenerative, Ca2+-dependent release of glutamate from astrocytes which, in turn, can enhance excitatory synaptic transmission via N-methyl-d-aspartate receptor-mediated mechanisms (9). Glutamate-mediated astrocyte-to-neuron signaling has also been observed in hippocampal slices (23), visual cortical slices (8), and the retina (10), although the subclass of the responsible glutamate receptors varied among the different preparations. We find, however, that in hippocampal neurons ATP differs from glutamate as a signaling molecule between astrocytes and neurons in that it inhibits rather than potentiates synaptic transmission. Spontaneous Ca2+ oscillations in hippocampal neurons mediated by glutamatergic synaptic transmission (17, 24) decreased in frequency after the stimulation of Ca2+ waves in an ATP-dependent manner. We hypothesize that the opposing actions of glutamate and ATP released from astrocytes represent a means by which astrocytes can dynamically modulate neuronal activity by releasing distinct transmitters that can either excite or inhibit synaptic transmission. Whereas astrocytes are known to release glutamate via soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)-dependent vesicular mechanisms (25), it has recently been shown that the release of ATP is mediated by secretory mechanisms distinct from those of glutamate release (26, 27). That astrocytes release ATP and glutamate through different mechanisms supports the concept that astrocytes can exert divergent effects on synaptic transmission by releasing different neurotransmitters. Furthermore, the nature of the signal released from astrocytes may differ under varying physiological and pathological conditions (26).
In addition to mediating inhibitory rather than excitatory effects on synaptic transmission, ATP-mediated astrocyte-to-neuron signaling further differs from glutamate-dependent signaling mechanisms by the fact that it occurs in a tonic fashion. Application of the ATP-degrading enzyme apyrase induced a potentiation of spontaneous neuronal Ca2+ oscillations in the absence of any astrocytic stimulation, suggesting the presence of a constitutive ATP-dependent inhibition of synaptic transmission. Furthermore, by means of confocal microscopy we observed spontaneous astrocytic Ca2+ responses in both purified astrocyte cultures and mixed cultures of astrocytes and neurons. The spontaneous Ca2+ signals in astrocytes were inhibited by apyrase but persisted in the presence of TTX. Therefore, astrocytes constitutively release ATP in the absence of neuronal activity that exerts tonic down-regulation of excitatory synaptic transmission. ATP mediates astrocytic Ca2+ waves and can evoke neuronal Ca2+ responses in various parts of the CNS, such as the habenula (28), suggesting that ATP may be a ubiquitous mediator of astrocyte-to-neuron signaling implicated in the modulation of synaptic activity. Such a tonic modulation by astrocytic ATP might be a mechanism by which neurons tune their communications in the CNS.
The ATP receptor subtype(s) implicated in ATP-mediated inhibition of synaptic transmission in hippocampal neurons remains unknown. Although apyrase abolished the ATP-mediated inhibition, the nonselective P2 receptor antagonists suramin and 4-[[4-formyl-5-hydroxy-6-methyl-3-[(phosphonooxy)methyl]-2-pyridinyl]azo]-1,3-benzenedisulfonic acid (PPADS) were only able to partially attenuate the effects of exogenously applied ATP (17). Similarly, these antagonists only slightly affected the decrease in neuronal Ca2+ oscillations evoked by mechanical stimulation of an astrocyte (mechanical stimulation alone, 77 ± 10% decrease, n = 77; +PPADS, 68 ± 11% decrease, n = 11; +suramin, 72 ± 10% decrease, n = 11). Adenosine, a metabolite of ATP, is also involved in the inhibitory action via adenosine A1 receptors (17, 29). Thus, released ATP might exhibit its inhibitory action by being metabolized into adenosine. However, we (17) and others (30, 31) have already shown that the ATP-evoked inhibition did not disappear even in the presence of several antagonists to adenosine receptors, A1 receptors, or adenosine deaminase in hippocampal neurons. In addition, the effect of astrocytic ATP on the synaptic transmission almost disappeared in the presence of apyrase (grade III), which degrades ATP and ADP into ADP and AMP, respectively, but does not affect the metabolism of adenosine. These findings suggest that ATP itself is involved in the inhibition of synaptic transmission. Recently, the oligomeric association of A1 receptors with P2Y1 receptors (A1/P2Y1 receptors) generating A1 with P2Y1 receptor-like agonistic pharmacology has been reported (32). Such an oligomeric association occurs in hippocampal neurons (33), and the pharmacological characteristics of A1/P2Y1 receptors are similar to those involved in the inhibition of neuronal Ca2+ oscillations that we have reported (17). However, we must await the discovery of specific antagonists for such oligomeric A1/P2Y1 receptors to determine whether they are implicated in ATP-mediated inhibition of neuronal activity.
In conclusion, we have demonstrated that both tonic and activity-dependent release of ATP from astrocytes down-regulate excitatory synaptic transmission in hippocampal cultures. ATP therefore serves as a signaling molecule between astrocytes and neurons implicated in the modulation of synaptic transmission.
Acknowledgments
We thank Tomoko Obama for technical assistance, Ken Nakazawa for allowing us to use the Bio-Rad confocal microscopy, Yasuo Ohno for continuous encouragement, and Sami Fam for critical reading of the manuscript. This work was partly supported by The Organization for Pharmaceutical Safety and Research (Medical Frontier Project, MF-16), The Health Science Foundation of Japan, and a grant-in-aid for scientific research from the Ministry of Education, Science, Sports, and Culture of Japan.
Abbreviations: CCD, charge-coupled device; TTX, tetrodotoxin.
References
- 1.Haydon, P. G. (2001) Nat. Rev. Neurosci. 2, 185–193. [DOI] [PubMed] [Google Scholar]
- 2.Newman, E. A. & Zahs, K. R. (1997) Science 275, 844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guthrie, P. B., Knappenberger, J., Segal, M., Bennett, M. V., Charles, A. C. & Kater, S. B. (1999) J. Neurosci. 19, 520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fam, S. R., Gallagher, C. J. & Salter, M. W. (2000) J. Neurosci. 20, 2800–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parpura, V., Basarsky, T. A., Liu, F., Jeftinija, K., Jeftinija, S. & Haydon, P. G. (1994) Nature 369, 744–747. [DOI] [PubMed] [Google Scholar]
- 6.Nedergaard, M. (1994) Science 263, 1768–1771. [DOI] [PubMed] [Google Scholar]
- 7.Bezzi, P., Carmignoto, G., Pasti, L., Vesce, S., Rossi, D., Rizzini, B. L., Pozzan, T. & Volterra, A. (1998) Nature 391, 281–285. [DOI] [PubMed] [Google Scholar]
- 8.Pasti, L., Volterra, A., Pozzan, T. & Carmignoto, G. (1997) J. Neurosci. 17, 7817–7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Araque, A., Parpura, V., Sanzgiri, R. P. & Haydon, P. G. (1998) Eur. J. Neurosci. 10, 2129–2142. [DOI] [PubMed] [Google Scholar]
- 10.Newman, E. A. & Zahs, K. R. (1998) J. Neurosci. 18, 4022–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salter, M. W. & Hicks, J. L. (1994) J. Neurosci. 14, 1563–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cotrina, M. L., Lin, J. H., Alves-Rodrigues, A., Liu, S., Li, J., Azmi-Ghadimi, H., Kang, J., Naus, C. C. & Nedergaard, M. (1998) Proc. Natl. Acad. Sci. USA 95, 15735–15740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotrina, M. L., Lin, J. H., Lopez-Garcia, J. C., Naus, C. C. & Nedergaard, M. (2000) J. Neurosci. 20, 2835–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens, B. & Fields, R. D. (2000) Science 287, 2267–2271. [DOI] [PubMed] [Google Scholar]
- 15.Nakazawa, K., Inoue, K., Watano, T. & Koizumi, S. (1995) J. Physiol. 484, 447–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koizumi, S., Saito, Y., Nakazawa, K., Nakajima, K., Sawada, J. I., Kohsaka, S., Illes, P. & Inoue, K. (2002) Life Sci. 72, 431–442. [DOI] [PubMed] [Google Scholar]
- 17.Koizumi, S. & Inoue, K. (1997) Br. J. Pharmacol. 122, 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koizumi, S., Bootman, M. D., Bobanovic, L. K., Schell, M. J., Berridge, M. J. & Lipp, P. (1999) Neuron 22, 125–137. [DOI] [PubMed] [Google Scholar]
- 19.Koizumi, S., Rosa, P., Willars, G. B., Challiss, R. A., Taverna, E., Francolini, M., Bootman, M. D., Lipp, P., Inoue, K., Roder, J. & Jeromin, A. (2002) J. Biol. Chem. 277, 30315–30324. [DOI] [PubMed] [Google Scholar]
- 20.Wang, Z., Haydon, P. G. & Yeung, E. S. (2000) Anal. Chem. 72, 2001–2007. [DOI] [PubMed] [Google Scholar]
- 21.Kajihata, T., Kuroki, T., Ueki, H., Motomura, K., Nakahara, T. & Tashiro, N. (2001) in Monitoring Molecules in Neuroscience, ed. O'Connor, W. T. (Univ. Coll. Dublin Press, Dublin), pp. 401–402.
- 22.Reuter, H. & Porzig, H. (1995) Neuron 15, 1077–1084. [DOI] [PubMed] [Google Scholar]
- 23.Kang, J., Jiang, L., Goldman, S. A. & Nedergaard, M. (1998) Nat. Neurosci. 1, 683–692. [DOI] [PubMed] [Google Scholar]
- 24.Ogura, A., Iijima, T., Amano, T. & Kudo, Y. (1987) Neurosci. Lett. 78, 69–74. [DOI] [PubMed] [Google Scholar]
- 25.Jeftinija, S. D., Jeftinija, K. V., Stefanovic, G. & Liu, F. (1996) J. Neurochem. 66, 676–684. [DOI] [PubMed] [Google Scholar]
- 26.Coco, S., Calegari, F., Pravettoni, E., Pozzi, D., Taverna, E., Rosa, P., Matteoli, M. & Verderio, C. (2002) J. Biol. Chem. 278, 1354–1362. [DOI] [PubMed] [Google Scholar]
- 27.Stout, C. E., Costantin, J. L., Naus, C. C. & Charles, A. C. (2002) J. Biol. Chem. 277, 10482–10488. [DOI] [PubMed] [Google Scholar]
- 28.Edwards, F. A., Gibb, A. J. & Colquhoun, D. (1992) Nature 359, 144–147. [DOI] [PubMed] [Google Scholar]
- 29.Cunha, R. A., Sebastiao, A. M. & Ribeiro, J. A. (1998) J. Neurosci. 18, 1987–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koch, H., Kugelgen, I. & Starke, K. (1997) Naunyn-Schmiedeberg's Arch. Pharmacol. 355, 707–715. [DOI] [PubMed] [Google Scholar]
- 31.Mendoza-Fernandez, V., Andrew, R. D. & Barajas-Lopez, C. (2000) J. Pharmacol. Exp. Ther. 293, 172–179. [PubMed] [Google Scholar]
- 32.Yoshioka, K., Saitoh, O. & Nakata, H. (2001) Proc. Natl. Acad. Sci. USA 98, 7617–7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshioka, K., Hosoda, R., Kuroda, Y. & Nakata, H. (2002) FEBS Lett. 531, 299–303. [DOI] [PubMed] [Google Scholar]