Abstract
Abscisic acid (ABA) triggers a complex sequence of signaling events that lead to concerted modulation of ion channels at the plasma membrane of guard cells and solute efflux to drive stomatal closure in plant leaves. Recent work has indicated that nitric oxide (NO) and its synthesis are a prerequisite for ABA signal transduction in Arabidopsis and Vicia guard cells. Its mechanism(s) of action is not well defined in guard cells and, generally, in higher plants. Here we show directly that NO selectively regulates Ca2+-sensitive ion channels of Vicia guard cells by promoting Ca2+ release from intracellular stores to raise cytosolic-free [Ca2+]. NO-sensitive Ca2+ release was blocked by antagonists of guanylate cyclase and cyclic ADP ribose-dependent endomembrane Ca2+ channels, implying an action mediated via a cGMP-dependent cascade. NO did not recapitulate ABA-evoked control of plasma membrane Ca2+ channels and Ca2+-insensitive K+ channels, and NO scavengers failed to block the activation of these K+ channels evoked by ABA. These results place NO action firmly within one branch of the Ca2+-signaling pathways engaged by ABA and define the boundaries of parallel signaling events in the control of guard cell movements.
Keywords: cGMP-mediated signaling, stress physiology, cyclic ADP ribose, cytosolic-free [Ca2+] elevation, Vicia
The gas nitric oxide (NO) is a highly reactive free radical that serves as a cellular signaling molecule. It is a byproduct of several cellular reactions and a natural constituent of all living cells, but its functions are known principally from studies of mammalian physiology (1–3). In animals, NO acts indirectly through guanylate cyclase to activate cGMP-dependent cellular responses and directly through S-nitrosylation of elements down-stream of several signal cascades (3). Among others, NO affects the gating of Ca2+-dependent K+ channels, Ca2+, and Na+ channels (4–6).
Evidence increasingly points to a role for NO also in plant development, stress responses, and programmed cell death (7, 8), although its situation within any one signal cascade is still poorly understood. NO action on stomatal guard cells is a case in point. Guard cells open and close the stoma of higher-plant leaves to balance gas exchange for photosynthesis against water loss via transpiration (9–11). NO enhances plant tolerance to drought (7) and contributes to stomatal closure evoked by the water-stress hormone abscisic acid (ABA). In plants, NO is produced from NO2 through photoconversion by carotenoids, reaction with nitrate reductases (NRs) (7, 12), and with glycine decarboxylase (13). In NR-deficient Arabidopsis, stomata fail to close in ABA (14). Furthermore, NO scavengers suppress ABA action in closing stomata, and NO donors promote closure in the absence of ABA (15, 16). However, NR-deficient Arabidopsis does not show a wilty phenotype (14). Thus, although NO seems to play a role in water-stress signaling, its situation within ABA-related signaling pathways and its relationship to ion transport that drives stomatal movement has remained unclear.
ABA closes stomata by regulating guard cell membrane transport to promote osmotic solute loss. Among its actions, ABA raises cytosolic-free [Ca2+] ([Ca2+]i) and cytosolic pH (pHi); these signals inactivate inward-rectifying K+ channels (IK,in) to prevent K+ uptake and activate outward-rectifying K+ channels (IK,out) and Cl- (anion) channels (ICl) at the plasma membrane to facilitate solute efflux (9, 10, 17). To explore NO function in guard cells and its association with ABA signal transduction, we recorded guard cell membrane current under voltage clamp and [Ca2+]i using fura 2 fluorescence ratio imaging. Our results demonstrate that NO promotes intracellular Ca2+ release and thereby regulates guard cell ion channels via a subset of signaling pathways enlisted by ABA.
Materials and Methods
Plant Material and Electrophysiology. Protoplasts and epidermal strips were prepared from Vicia faba L., and operations were carried out on a Zeiss Axiovert microscope with ×63 long working distance differential interference contrast microscopy optics (18, 19). Patch pipettes were pulled with a Narashige (Tokyo) PP-83 puller, and currents were recorded and analyzed as described (18, 20). Voltage-clamp recordings and fura 2 injections of intact guard cells were carried out by impalement with two- and three-barrelled microelectrodes (19, 20).
[Ca2+]i Measurements. [Ca2+]i was determined by fura 2 fluorescence ratio imaging with a GenIV-intensified Pentamax-512 charge-coupled device camera (Princeton Instruments, Trenton, NJ) (20). Measurements were corrected for background before loading and analyzed with Universal Imaging software (Media, PA). Fura 2 fluorescence was calibrated in vitro and in vivo after permeabilization (19). Estimates of loading indicated final fura 2 concentrations <10 μM (19).
Numerical Analysis. Currents from intact cells were recorded and analyzed with HENRY II software (Y-Science, Glasgow, U.K., www.gla.ac.uk/ibls/BMB/mrb/lppbh.htm). Channel amplitudes were calculated from point-amplitude histograms of openings >5 ms in duration beyond closed levels, and channel number, openings, and probabilities were determined as described (18, 20). Results are reported as means ± SE.
Chemicals and Solutions. Intact cells were bathed in 5 mM Ca-Mes, pH 6.1 [Mes titrated to its pKa with Ca(OH)2] with 10 mM KCl or 15 mM CsCl/15 mM tetraethylammonium-Cl to verify Cl- currents (21). Protoplasts were bathed in Ba2+-Hepes, pH 7.5 [Hepes buffer titrated to its pKa with Ba(OH)2] adjusted to 300 milliosmolar with sorbitol, and pipettes were filled with similar solutions. For cell-attached recording, pipette and bath contained 30 mM Ba2+; for whole-cell recording, pipettes contained 1 mM Ba2+ and (Mg2+)2ATP, and the bath contained 30 mM Ba2+; and for excised, inside-out patches, pipettes contained 30 mM Ba2+, and the bath contained 1 mM Ba2+ and (Mg2+)2ATP. S-nitroso-N-acetyl-penacillamine (SNAP) was dissolved in 1:1 ethanol/H2O, and 1-H-(1,2,4)-oxadiazole-[4,3-a]quinolxalin-1-one (ODQ) was dissolved in DMSO before 1,000-fold dilution for use. Ethanol and DMSO alone at this concentration had no effect (18, 19). Sodium nitroprusside (SNP), the NO scavenger 2-carboxyphenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO), and all other compounds were used directly. NO generated from 10 μM SNP and SNAP was determined via metahemoglobin formation (22) to yield 10 nM/min. All reagents were from Sigma or Calbiochem.
Results
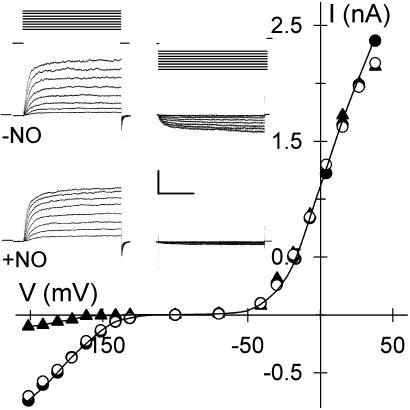
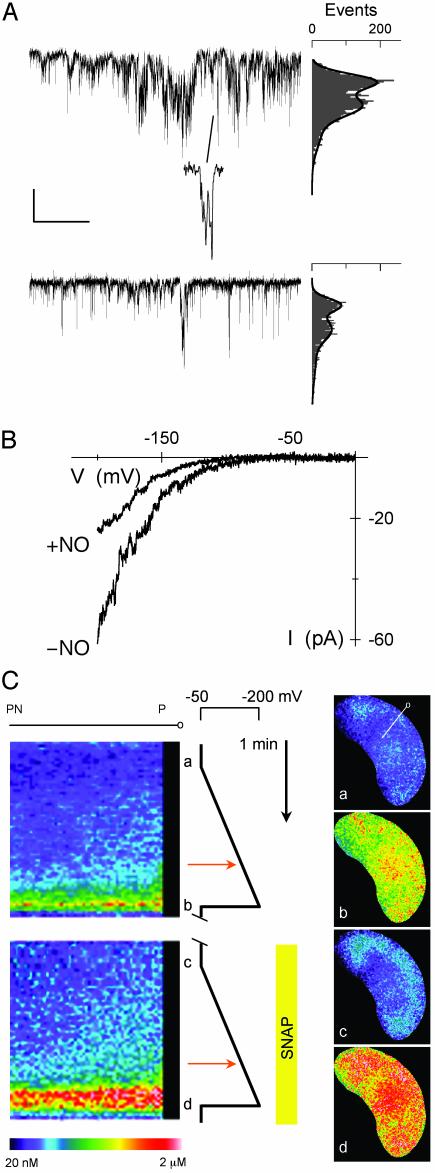
NO Targets IK,in and ICl. We used the membrane-permeant NO donors SNAP and SNP and the NO scavenger cPTIO while recording membrane current from intact Vicia guard cells under voltage clamp. Fig. 1 shows current traces and steady-state current–voltage curves from one guard cell recorded before and after a 60-s exposure to 10 μM SNAP, yielding 10 nM NO per min. Voltage steps positive of -50 mV were marked by an outward current, typical of IK,out, that relaxed to a new steady state with half-times near 300 ms; steps negative of -120 mV gave an inward current characteristic of IK,in. SNAP exposure dramatically reduced the amplitude of IK,in. This response was complete within 60 s, and the effect was reversed after washing for 2 min with 20 μM cPTIO. IK,out was not affected by the NO donor at this concentration even after 10 min of exposure. Similar results were obtained in 27 experiments with SNAP (see Fig. 2) and with 10 μM SNP (n = 22; data not shown). IK,in inactivation was reversed either after adding cPTIO or after 4–6 min of washing in buffer alone. NO treatment also led to a reversible, 2.1-± 0.4-fold increase in background current (Fig. 2) identified with ICl at the plasma membrane (21, 23), indicating a significant rise in ICl in response to NO.
Fig. 1.
NO selectively inactivates IK,in. Voltage-clamp recordings from an intact Vicia guard cell are shown. (Inset) Steady-state current–voltage curves determined from voltage-clamp steps before (•), after 2 min of exposure to 10 μM SNAP (▴), and after 6 min of washing in buffer-SNAP (○). K+-channel currents were obtained by subtracting instantaneous current from steady-state current at each voltage. Data for IK,in and IK,out were fitted jointly (solid lines) to common Boltzmann functions (20, 24) with the voltage sensitivity parameter V1/2, the voltage giving half-maximal conductance, free to vary between curves. Values for V1/2 (IK,in): -NO, -173 ± 4 mV; +NO, -192 ± 9 mV. (Inset) Current traces for IK,out (Left) and IK,in (Right) before (Middle) and during (Bottom) NO treatment. Zero current is indicated on the left. Voltage protocols (Top) of steps between -200 and +50 mV from holding voltage of -100 mV are shown. (Scale: horizontal, 2 s; vertical, 1 nA.)
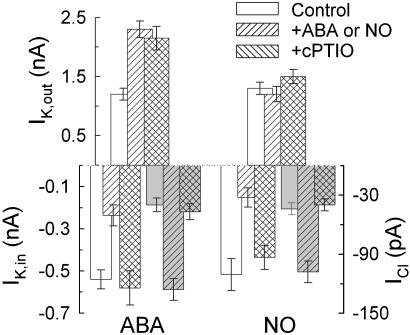
Fig. 2.
NO scavenger cPTIO blocks inactivation of IK,in and activation of ICl by ABA and NO but not ABA-mediated activation of IK,out. Steady-state current determined as described for Fig. 1 for IK,out at +30 mV, IK,in at -200 mV, and ICl at -70 mV (shaded bars) (21) summarizes effects of a 10-min exposure to ABA with (n = 9) or without 20 μM cPTIO (n = 8) (Left) and to 10 μM SNAP with (n = 7) or without 20 μM cPTIO (n = 27) (Right).
We determined half-times for current activation from guard cells for which IK,in could be resolved in NO. Mean half-times at -200 mV were 314 ± 35 ms before treatments. After 2 min in SNAP and SNP, half-times were 538 ± 72 (n = 8) and 593 ± 85 (n = 6), respectively, indicating a substantial change in channel gating. Fitting current–voltage curves before and during NO treatments jointly to a Boltzmann function showed a change in voltage sensitivity, thus supporting this conclusion. Fittings probably underestimated the change in voltage sensitivity with NO, because data points were restricted to voltages at or greater than -200 mV. Even so, best fittings gave half-maximal activation voltages, V1/2, displaced by ≥18 mV negative in NO (Fig. 1). Thus, similar to the effect of ABA (11), inactivation of IK,in by NO entailed changes in IK,in gating.
Because NO scavengers suppress ABA-evoked stomatal closing (15, 16), we were interested to determine whether this action, similar to that of the NO donors, was selective for IK,in and ICl over IK,out. We recorded membrane currents before and after adding 10 μM ABA either in the presence or absence of 20 μM cPTIO. The results (Fig. 2) showed that the NO scavenger prevented IK,in inactivation and ICl activation but was without influence on the increase in IK,out evoked by ABA (11). These results discounted any general inhibitory effects of the NO donors or cPTIO and prompted us to examine signal cascades associated specifically with the inward-rectifying K+ channels.
IK,in Inactivation and ICl Activation by NO Requires Elevated [Ca2+]i. Guard cell K+ channels are known to be controlled by at least two separate signaling pathways in ABA. IK,in and ICl respond to increases in [Ca2+]i (9–11). Effects on IK,in of elevating [Ca2+]i include a slowing of the current kinetics and negative shift in its voltage sensitivity. IK,out is [Ca2+]i-insensitive and is regulated instead by a slower, ABA-evoked rise in pHi (10, 11). These parallels led us to explore NO action in elevating [Ca2+]i.
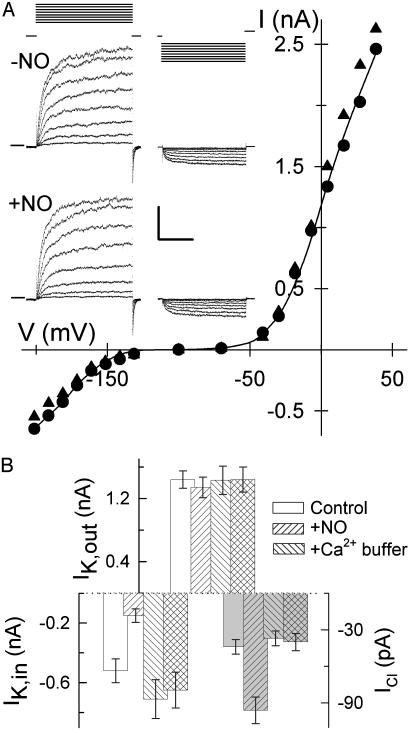
We suppressed changes in [Ca2+]i by loading guard cells from microelectrodes containing Ca2+ buffer, either 50 mM EGTA (n = 2) or 50 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (n = 8), before adding SNAP (Fig. 3). Alone, Ca2+ buffering led to a small but not very significant rise in IK,in amplitude, consistent with the low Ca2+ sensitivity of IK,in below 150 nM [Ca2+]i (24). Adding NO thereafter showed that the inactivation of IK,in and activation of ICl were suppressed almost entirely. Thus, elevated [Ca2+]i is an essential precondition to transmitting the NO signal.
Fig. 3.
Ca2+ buffering blocks NO inactivation of IK,in and activation of ICl. (A) Voltage-clamp recordings from an intact Vicia guard cell impaled and buffer-loaded from a microelectrode containing 50 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (BAPTA). Shown are steady-state current–voltage curves determined from voltage-clamp steps (Inset) before (•) and after 6 min of exposure to 10 μM SNAP (▴) as described for Fig. 1. Data for IK,in and IK,out fitted jointly (solid lines) to common Boltzmann functions (see Fig. 1) gave half-maximal conductance for IK,in of -178 ± 3mV(-NO) and -181 ± 4mV(+NO). (Inset) Current traces for IK,out (Left) and IK,in (Right) before (Middle) and during (Bottom) NO treatment. Zero current is indicated on the left. Voltage protocols (Top) of steps between -200 mV and +50mV from holding voltage of -100 mV. (Scale: horizontal, 2 s; vertical, 1 nA.) (B) Summary of IK,out, IK,in, and ICl (shaded bars) before and after exposure to 10 μM SNAP with (n = 10) and without (n = 27) 50 mM EGTA or 50 mM BAPTA loading. Currents were determined as described for Figs. 1 and 2.
NO Elevates Resting and Voltage-Evoked [Ca2+]i Increases. To determine whether NO affected [Ca2+]i, we loaded guard cells with the Ca2+-sensitive dye fura 2 to quantify [Ca2+]i by fluorescence ratio imaging. Ca2+ channels at the Vicia guard cell plasma membrane activate on hyperpolarization, which leads to Ca2+ influx and Ca2+-induced Ca2+ release from intracellular stores (18, 19), so resting [Ca2+]i was determined with the cells clamped to -50 mV. Increases in [Ca2+]i were evoked by 20-s voltage steps and 90-s voltage ramps between -50 mV and -200 mV. Fluorescence from the entire cell was used to quantify the overall [Ca2+]i rise and recovery. Fluorescence from a 2-μm band around the cell periphery was used to determine the voltage threshold initiating [Ca2+]i increases (19, 20).
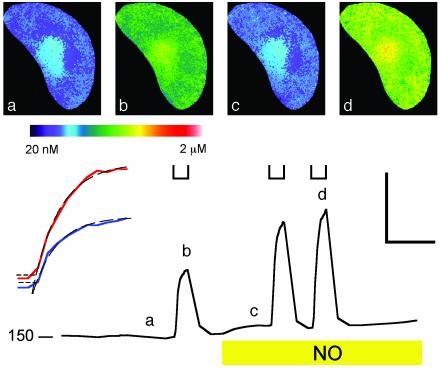
Fig. 4 shows [Ca2+]i from one guard cell before and after adding SNAP. Resting [Ca2+]i was close to 160 nM. Stepping the voltage to -200 mV evoked a characteristic [Ca2+]i elevation to >500 nM, and [Ca2+]i recovered over 1–2 min after returning to -50 mV. Maximal [Ca2+]i was generally more pronounced near the perinuclear region, reflecting the high density of endoplasmic reticulum around the nucleus (see refs. 19 and 20). Adding NO led to an increase in resting [Ca2+]i, and after 2 min in NO, clamp steps to -200 mV evoked [Ca2+]i increases to near 800 nM. Similar results were obtained in five other experiments, with NO treatment giving a significant rise in resting [Ca2+]i and an enhanced response to hyperpolarizing voltage steps (Table 1). However, NO did not alter [Ca2+]i recovery noticeably after voltage steps (Fig. 4).
Fig. 4.
NO promotes evoked [Ca2+]i increases without affecting [Ca2+]i recovery. (Lower) [Ca2+]i recorded from one guard cell clamped to -50 mV and stepped to -200 mV at time periods indicated ([unionsq]) before and after adding 10 μM SNAP show enhanced [Ca2+]i rise but no apparent change in recovery kinetics at -50 mV. (Lower Left) [Ca2+]i basal level in nM. Fura 2 fluorescence images taken at 2-s intervals. (Upper) Selected ratio images (a–d) correspond to the time points indicated. (Lower Left Inset) Expanded analysis of [Ca2+]i rise from 2 s before -200 mV steps. -NO (blue) and +NO (red) show no change in lag time. The data points above +1 SD from mean [Ca2+]i before clamp step (horizontal dashed lines) fitted to single exponentials gave equivalent lag periods and rise half-times of 4.2 s despite the difference in amplitudes. [Scale: horizontal, 1 min (Lower Left Inset, 10 s); vertical 400 nM.]
Table 1. Guanylate cyclase and cADPR antagonists suppress NO inactivation of IK,in and [Ca2+]i elevation.
| Treatment | IK,in (pA) | [Ca2+]i resting (nM) | [Ca2+]i evoked (nM) |
|---|---|---|---|
| Control | -517 ± 53 (27) | 159 ± 15 (6) | 452 ± 31 (6) |
| 10 μM SNAP | -151 ± 35 (27) | 262 ± 31 (6) | 741 ± 70 (6) |
| +1 μM ryanodine* | -535 ± 72 (5) | 171 ± 11 (3) | 200 ± 12 (3) |
| +20 μM ODQ | -443 ± 36 (5) | 106 ± 26 (4) | 303 ± 68 (4) |
Because Ca2+ must first cross the plasma membrane to trigger Ca2+ release and global [Ca2+]i elevation, its kinetics provides a rough guide to distinguish between effects on Ca2+ entry and intracellular Ca2+ release. We fitted data recorded during steps to an exponential function to determine the rate of [Ca2+]i rise within the cell and calculated the time for [Ca2+]i to rise 1 SD above the mean resting [Ca2+]i to estimate the delay to intracellular Ca2+ release. The analysis (Fig. 4 Inset) showed no difference in lag time to the onset of [Ca2+]i increases before and after adding NO (lag times: -NO, 1.3 ± 0.1 s; +NO, 1.4 ± 0.1 s; n = 5). However, NO led to an ≈2-fold increase in the rate of [Ca2+]i rise, from 42 ± 15 nM/s before to 86 ± 16 nM/s after adding SNAP (n = 5). These results suggested a predominant effect after intracellular Ca2+ release rather than after Ca2+ entry.
NO Does Not Promote Ca2+-Channel Gating at the Plasma Membrane. To assess the effect of NO on Ca2+ channels at the plasma membrane directly, we recorded whole-cell and single-Ca2+ channel currents from guard cell protoplasts. Measurements were carried out with Ba2+ as the charge carrier and to block K+ channels, and Cl- was omitted from all solutions to avoid a background of Cl--channel activity (18, 20). In both cell-attached (Fig. 5A) and excised patches (data not shown), clamping to -150 mV yielded short, flickering events of 1.8 ± 0.2 pA (13 ± 1 pS, n = 6) characteristic of the Ca2+ channel (18, 20). No effect on single-channel conductance was observed, but adding 10 μM SNAP led to a 1.7-± 0.5-fold reduction in the number of openings (Fig. 5A Right) and in single-channel activity, NPo (-SNAP, 0.104 ± 0.004; +SNAP, 0.049 ± 0.003; n = 6). Whole-cell current recorded during voltage ramps (Fig. 5B) showed a 1.9-± 0.4-fold (n = 6) decline in current that stabilized within 2 min in NO and was fully reversible after washing NO from the bath. This effect was scalar and showed no evidence of a shift in the voltage sensitivity for Ca2+-channel gating that is characteristic of ABA (18).
Fig. 5.
NO enhances evoked [Ca2+]i increases without promoting Ca2+-channel activity at the plasma membrane. (A) Cell-attached, single Ca2+-channel records from one guard cell protoplast before (Top) and 2 min after (Bottom) adding 10 μM SNAP. (Middle) Expanded time scale showing single opening events. [Scale: horizontal, 1 s (50 ms, Middle); vertical, 2 pA.] Point-amplitude histograms (Right), which plot the number of openings and their mean amplitude (the vertical axis is scaled to traces) for 10-s periods, including segments shown, indicate an ≈2-fold decrease in opening events. (B) Whole-cell Ca2+ currents recorded during voltage ramps before and 2 min after adding 10 μM SNAP (see ref. 18). (C) Kymograph (Left) of voltage evoked [Ca2+]i rise in one intact guard cell recorded by fura 2 fluorescence ratio at 2-s intervals before and 2 min after adding 10 μM SNAP. The time line (Center) runs top to bottom with voltage scale and ramps as indicated. Selected ratio images (Right,a–d) correspond to time points indicated. The kymograph was constructed from successive ratio images averaged over a 2-pixel-wide band (line in Right, a) from cell exterior and periphery (P) to the perinuclear region (PN). Voltage ramps from -50 mV to -200 mV over 90 s. The threshold for [Ca2+]i rise was determined as time of [Ca2+]i rise 1 SD above the pre-ramp level to a depth of 3 pixel units (≈2 μm). Thresholds (red arrows, Center): -NO, -138 mV; +SNAP, -143 mV.
To relate NO action on the Ca2+ channel with evoked [Ca2+]i increases, we determined the voltage threshold for [Ca2+]i rise during voltage ramps in intact guard cells. Fig. 5C shows data from one guard cell. Individual ratio images are shown (Right, a–d) corresponding to time points indicated along the voltage timeline (Center). The kymograph (Left) shows the kinetics of the [Ca2+]i rise for a band stretching from the cell periphery (P) to the perinuclear (PN) region (tagged line in a, upper right). Again, a rise in resting [Ca2+]i and evoked [Ca2+]i increases was seen in NO. However, no difference was found in the voltage threshold evoking the [Ca2+]i rise in NO (Center, red arrows). Indeed, on a cell-by-cell basis NO led to a reproducible negative shift in voltage evoking the [Ca2+]i rise consistent with its action on the plasma membrane Ca2+ channels, although statistically the difference in the pooled data was not significant (-NO, -133 ± 7 mV; +NO, -145 ± 6 mV; n = 7). Thus, NO must promote [Ca2+]i elevation entirely by enhancing intracellular Ca2+ release.
[Ca2+]i Rise and IK,in Inactivation Are Sensitive to Antagonists of Guanylate Cyclase and Cyclic ADP Ribose (cADPR)-Mediated Ca2+ Release. One mechanism for [Ca2+]i elevation relies on Ca2+ release via endomembrane Ca2+ channels that are activated by cADPR. In animals, cGMP stimulates cADPR biosynthesis to mobilize Ca2+ downstream of NO (3). Elements of one or more analogous pathways have been implicated in plant cells, including guard cells in ABA and Ca2+ signal processing (24, 25) and in NO (15), but their functional relationship has never been demonstrated directly.
To explore the mechanism for NO-enhanced Ca2+ release, we used ryanodine and ODQ, antagonists of cADPR-mediated Ca2+ release and of NO-sensitive guanylate cyclase, respectively, in animals that are also active in plants (15, 24, 26). Table 1 summarizes IK,in and [Ca2+]i measurements carried out as described above. Although several mechanisms for Ca2+ release are present in guard cells (9, 27), we found that 5-min pretreatments with ryanodine entirely suppressed [Ca2+]i increases and the associated inactivation of IK,in (data not shown), which is consistent with the predominance of cADPR/ryanodine-sensitive Ca2+ release in response to voltage-evoked Ca2+ entry. Thereafter, NO gave no enhancement in resting [Ca2+]i or evoked [Ca2+]i increases, nor did it inactivate IK,in. Pretreatment with ODQ also blocked NO action on [Ca2+]i and IK,in but only partially suppressed evoked [Ca2+]i increases and IK,in inactivation without NO. These results are consistent with a basal activity of ryanodine-sensitive Ca2+ release channels without enhanced cADPR synthesis and a requirement for NO-dependent guanylate cyclase activity and elevated cADPR levels to sensitize NO-dependent Ca2+ release.
Discussion
A growing body of data in recent years has linked NO to signaling in plants. Notably, genetic evidence of a prerequisite for NO synthesis in ABA-mediated stomatal control (14) has fueled debate about a link between NO, Ca2+-mediated signaling, and ion-channel regulation in guard cells. Until now, however, no direct evidence has been available to delineate NO action in these or other plant cell models. Our results above unequivocally establish [Ca2+]i as a principle target for NO in guard cells, demonstrating its action in promoting specifically intracellular Ca2+ release and, by elevating [Ca2+]i, in regulating Ca2+-senstive K+ and Cl- channels at the plasma membrane. We also report that NO does not recapitulate ABA in activating plasma membrane Ca2+ channels and outward-rectifying (Ca2+-insensitive) K+ channels, nor does the NO scavenger cPTIO block ABA action on these K+ channels. Thus, we unambiguously delimit the action of NO at low nanomolar concentrations to Ca2+ release from intracellular stores and, hence, to a subset of ABA-associated events and downstream ion-channel responses.
A key line of evidence to the targets for NO draws on the well documented separation of signals that regulate the two major K+ channels at the guard cell plasma membrane. ABA controls IK,out and IK,in in parallel, but its action on IK,in depends on an early rise in [Ca2+]i and only secondarily on the slower elevation in pHi in ABA (9, 11, 24). Elevated [Ca2+]i also activates ICl (ref. 10; see also Fig. 3). By contrast, IK,out is insensitive to [Ca2+]i, and its activation by ABA depends on pHi (11). Thus, NO action clearly aligned with that of ABA-evoked changes in [Ca2+]i, a point we demonstrated directly in subsequent measurements of [Ca2+]i.
Two important downstream components of NO signaling in animals, cGMP and cADPR, function in plants and have the potential to influence [Ca2+]i. One mode of cGMP action in animals is to promote the biosynthesis of cADPR, which stimulates ryanodine-sensitive Ca2+ channels at the endoplasmic reticulum and mobilizes Ca2+ release (3). cADPR is known to affect Ca2+ release from endomembrane stores in plants including the endoplasmic reticulum (28), and in guard cells, cADPR- and ryanodine-sensitive Ca2+ release contribute to ABA physiology and voltage-evoked [Ca2+]i increases (24, 25). A second mode of cGMP action entails binding and direct activation of cyclic nucleotide-gated ion channels (CNGCs). In animals, CNGCs are found at the plasma membrane and are active in visual, taste, and olfactory signal transduction (1, 29, 30). Less is known of plant CNGCs, but they seem to have roles in pathogen defense responses (31, 32). CNGCs have been suggested as a pathway for Ca2+ entry to the cytosol (15, 26), although substantive evidence is lacking. cGMP also affects Ca2+ sequestration through protein kinase-mediated phosphorylation that regulates Ca2+ ATPases (33). Finally, NO can S-nitrosylate the cysteines of ion channels including the cADPR/ryanodine-sensitive Ca2+ channel of skeletal muscle (34, 35) and epithelial K+ channels (5, 6). Thus NO is able to regulate ion channels directly as well as affect [Ca2+]i and, hence, Ca2+-sensitive signaling processes via at least four different pathways for which several components are documented in plants.
Our findings point to an action of NO on Ca2+ release through cADPR/ryanodine-sensitive Ca2+ channels. Inactivation of IK,in and activation of ICl were blocked in guard cells preloaded with Ca2+ buffers (Fig. 3), discounting a significant, [Ca2+]i-independent action on the channels. An effect on plasma membrane Ca2+ channels was ruled out in single-channel and whole-cell recordings and in measurements of evoked [Ca2+]i increases (Figs. 4 and 5). The same data argue against Ca2+ entry via NO-sensitive or rectifying (36) CNGCs or their inclusion as a significant component to the background current in NO. In plants, as in animals, most CNGCs are voltage-insensitive and nonselective (29, 31, 37), thus Ca2+ entry on activating these channels would depolarize the threshold for [Ca2+]i elevation. By contrast, NO enhanced resting [Ca2+]i and the rate and amplitude of evoked [Ca2+]i rise within guard cells, although [Ca2+]i recovered normally after voltage steps (Fig. 4). These results point to a sensitization of internal Ca2+ release but indicate that resequestration in Ca2+ stores was unaffected. Finally, ryanodine and ODQ, antagonists of cADPR-sensitive Ca2+ channels and guanylate cyclase, respectively, blocked NO action on IK,in and [Ca2+]i (Table 1).
How might the NO signal be transmitted? In animals, a subfamily of soluble guanylate cyclases are activated by NO (3). Plant guanylate cyclases are structurally different from the animal enzymes, and an NO sensitivity has yet to be identified (38). Nonetheless, cGMP has been unambiguously demonstrated in plants by mass spectrometry (39), and an NO-sensitive guanylate cyclase is implicated in several plant tissues including Arabidopsis and Vicia guard cells, for which the physiological effects of NO are suppressed by ODQ, an inhibitor of mammalian NO-sensitive guanylate cyclase (15, 26). Thus it seems that all the major elements of an NO-driven and Ca2+-coupled signal cascade occur and function in Vicia guard cells. We conclude that NO acts via cGMP synthesis and cADPR to sensitize endomembrane Ca2+ channels for Ca2+-induced Ca2+ release.
It is clear, too, that additional transduction pathways must operate in parallel in the guard cells. A comparison with the response to ABA is instructive. In addition to enhancing IK,out, ABA affects [Ca2+]i via three separate mechanisms (18–20, 40): (i) similar to NO, it accelerates Ca2+-induced Ca2+ release from intracellular stores; however, unlike NO, (ii) ABA alters the voltage sensitivity for gating of plasma membrane Ca2+ channels to promote Ca2+ entry across the plasma membrane, and (iii) it suppresses Ca2+ resequestration. As a consequence [Ca2+]i increases in ABA, unlike NO, are triggered at membrane voltages positive of -100 mV, and once elevated, [Ca2+]i recovers its previous resting level only over periods of ≥3–5 min.
Other reactive oxygen species, notably H2O2, have been hypothesized to play a central role in ABA signaling (41). At concentrations ≥50 μM, H2O2 affects Ca2+-channel gating at the guard cell plasma membrane and elevates [Ca2+]i (20, 40). However, unlike NO and ABA, exogenous H2O2 effects complete inactivation of both IK,in and IK,out, thus eliminating a major pathway for K+ efflux (20). By contrast, NO is permissive at low nanomolar concentrations: It engages only a subset of ABA-related events downstream that depend on elevated [Ca2+]i without a significant effect on IK,out (Fig. 1). Only with ≥100 nM NO was IK,out seen to inactivate reversibly (data not shown), which may indicate direct S-nitrosylation of the K+ channel or associated proteins (3, 42). In fact, many signaling pathways in animals originally thought to be activated by H2O2 actually may be controlled by other metabolites including NO (2). A requirement for NAD(P)H and reactive oxygen species in ABA signaling of guard cells (41) may be attributable at least in part to NO production by NADPH nitrate reductases (7, 14).
Finally, these findings give substance to the mechanism for stomatal closure in NO. Solute loss from the guard cells in ABA normally requires an increase in ICl, which drives the membrane positive of the K+ equilibrium voltage (EK) to activate IK,out and to balance charge for the K+ current. K+ uptake, in turn, is suppressed both by the voltage sensitivity of IK,in and through its strong inactivation by [Ca2+]i > 200 nM (24). In ABA, IK,out is enhanced by a rise in pHi, but IK,out carries an appreciable current positive of EK in the absence of ABA (Fig. 1). Thus, even at the elevated resting [Ca2+]i in NO (Fig. 4 and Table 1), IK,in will be suppressed. Thus, together with activation of ICl (Fig. 2), NO may be expected to favor solute loss and stomatal closure, albeit at a reduced rate compared with ABA (see Fig. 1 and ref. 15).
In conclusion, we show that NO selectively regulates the Ca2+-sensitive K+ and Cl- channels of Vicia guard cells by promoting Ca2+ release from intracellular stores to raise [Ca2+]i. Additional evidence points to NO action mediated by a cGMP-dependent cascade. Unlike ABA, low nanomolar concentrations of NO do not affect plasma membrane Ca2+ channels and Ca2+-insensitive K+ channels, nor does NO scavenging prevent ABA from activating these K+ channels. These results place NO action firmly within a subset of the signaling pathways enlisted by ABA.
Acknowledgments
We thank Anna Amtmann for comments on the manuscript. This work was supported by Biotechnology and Biological Sciences Research Council Grants P09640, C10234, and P09561 to M.R.B., Agencia Nacional de Promoción de Ciencia y Tecnología, and a Foundation Antorchas travel grant to L.L. and C.G.-M.
Abbreviations: ABA, abscisic acid; [Ca2+]i, cytosolic-free Ca2+ concentration; pHi, cytosolic pH; IK,in, inward-rectifying (Ca2+-sensitive) K+ channel (current); IK,out, outward-rectifying (Ca2+-insensitive) K+ channel (current); ICl, Cl- (anion) channel (current); SNAP, S-nitroso-N-acetyl-penacillamine; ODQ, 1-H-(1, 2, 4)-oxadiazole-[4,3-a]quinolxalin-1-one; SNP, sodium nitroprusside; cPTIO, 2-carboxyphenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide; cADPR, cyclic ADP ribose; CNGC, cyclic nucleotide-gated ion channel.
References
- 1.Gibson, A. D. & Garbers, D. L. (2000) Annu. Rev. Neurosci. 23, 417-439. [DOI] [PubMed] [Google Scholar]
- 2.Stamler, J. S. (1994) Cell 78, 931-936. [DOI] [PubMed] [Google Scholar]
- 3.Ahern, G. P., Klyachko, V. A. & Jackson, M. B. (2002) Trends Neurosci. 25, 510-517. [DOI] [PubMed] [Google Scholar]
- 4.Renganathan, M., Cummins, T. R. & Waxman, S. G. (2002) J. Neurophysiol. 87, 761-775. [DOI] [PubMed] [Google Scholar]
- 5.Tang, X. D., Daggett, H., Hanner, M., Garcia, M. L., Mcmanus, O. B., Brot, N., Weissbach, H., Heinemann, S. H. & Hoshi, T. (2001) J. Gen. Physiol. 117, 253-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolotina, V. M., Najibi, S., Palacino, J. J., Pagano, P. J. & Cohen, R. A. (1994) Nature 368, 850-853. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Mata, C. & Lamattina, L. (2003) Trends Plant Sci. 8, 20-26. [DOI] [PubMed] [Google Scholar]
- 8.Delledonne, M., Xia, Y. J., Dixon, R. A. & Lamb, C. (1998) Nature 394, 585-588. [DOI] [PubMed] [Google Scholar]
- 9.Hetherington, A. M. (2001) Cell 107, 711-714. [DOI] [PubMed] [Google Scholar]
- 10.Schroeder, J. I., Allen, G. J., Hugouvieux, V., Kwak, J. M. & Waner, D. (2001) Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 627-658. [DOI] [PubMed] [Google Scholar]
- 11.Blatt, M. R. (2000) Annu. Rev. Cell Dev. Biol. 16, 221-241. [DOI] [PubMed] [Google Scholar]
- 12.Rockel, P., Strube, F., Rockel, A., Wildt, J. & Kaiser, W. M. (2002) J. Exp. Bot. 53, 103-110. [PubMed] [Google Scholar]
- 13.Chandok, M. R., Ytterberg, A. J., van Wijk, K. J. & Klessig, D. F. (2003) Cell 113, 469-482. [DOI] [PubMed] [Google Scholar]
- 14.Desikan, R., Griffiths, R., Hancock, J. & Neill, S. (2002) Proc. Natl. Acad. Sci. USA 99, 16314-16318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neill, S. J., Desikan, R., Clarke, A. & Hancock, J. T. (2002) Plant Physiol. 128, 13-16. [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Mata, C. & Lamattina, L. (2002) Plant Physiol. 128, 790-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blatt, M. R. (2000) Curr. Opin. Plant Biol. 3, 196-204. [PubMed] [Google Scholar]
- 18.Hamilton, D. W. A., Hills, A., Kohler, B. & Blatt, M. R. (2000) Proc. Natl. Acad. Sci. USA 97, 4967-4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grabov, A. & Blatt, M. R. (1998) Proc. Natl. Acad. Sci. USA 95, 4778-4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Köhler, B. & Blatt, M. R. (2002) Plant J. 32, 185-194. [DOI] [PubMed] [Google Scholar]
- 21.Grabov, A., Leung, J., Giraudat, J. & Blatt, M. R. (1997) Plant J. 12, 203-213. [DOI] [PubMed] [Google Scholar]
- 22.Murphy, M. E. & Noack, E. (1994) Methods Enzymol. 233, 240-250. [DOI] [PubMed] [Google Scholar]
- 23.Pei, Z. M., Kuchitsu, K., Ward, J. M., Schwarz, M. & Schroeder, J. I. (1997) Plant Cell 9, 409-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grabov, A. & Blatt, M. R. (1999) Plant Physiol. 119, 277-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leckie, C. P., McAinsh, M. R., Allen, G. J., Sanders, D. & Hetherington, A. M. (1998) Proc. Natl. Acad. Sci. USA 95, 15837-15842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durner, J., Wendehenne, D. & Klessig, D. F. (1998) Proc. Natl. Acad. Sci. USA 95, 10328-10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanders, D., Pelloux, J., Brownlee, C. & Harper, J. F. (2002) Plant Cell 14, S401-S417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navazio, L., Mariani, P. & Sanders, D. (2001) Plant Physiol. 125, 2129-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finn, J. T., Grunwald, M. E. & Yau, K. W. (1996) Annu. Rev. Physiol. 58, 395-426. [DOI] [PubMed] [Google Scholar]
- 30.Zagotta, W. N. & Siegelbaum, S. A. (1996) Annu. Rev. Neurosci. 19, 235-263. [DOI] [PubMed] [Google Scholar]
- 31.Balague, C., Lin, B. Q., Alcon, C., Flottes, G., Malmstrom, S., Kohler, C., Neuhaus, G., Pelletier, G., Gaymard, F. & Roby, D. (2003) Plant Cell 15, 365-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clough, S. J., Fengler, K. A., Yu, I. C., Lippok, B., Smith, R. K. & Bent, A. F. (2000) Proc. Natl. Acad. Sci. USA 97, 9323-9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen, J., Wang, Y. P., Wang, Y., Nakajima, T., Iwasawa, K., Hikiji, H., Sunamoto, M., Choi, D. K., Yoshida, Y., Sakaki, Y., et al. (2000) J. Biol. Chem. 275, 28739-28749. [DOI] [PubMed] [Google Scholar]
- 34.Eu, J. P., Sun, J. H., Xu, L., Stamler, J. S. & Meissner, G. (2000) Cell 102, 499-509. [DOI] [PubMed] [Google Scholar]
- 35.Xu, L., Eu, J. P., Meissner, G. & Stamler, J. S. (1998) Science 279, 234-237. [DOI] [PubMed] [Google Scholar]
- 36.Leng, Q., Mercier, R. W., Hua, B. G., Fromm, H. & Berkowitz, G. A. (2002) Plant Physiol. 128, 400-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leng, Q., Mercier, R. W., Yao, W. Z. & Berkowitz, G. A. (1999) Plant Physiol. 121, 753-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ludidi, N. & Gehring, C. (2003) J. Biol. Chem. 278, 6490-6494. [DOI] [PubMed] [Google Scholar]
- 39.Brown, E. G. & Newton, R. P. (1992) Phytochem. Anal. 3, 1-13. [Google Scholar]
- 40.Pei, Z. M., Murata, Y., Benning, G., Thomine, S., Klusener, B., Allen, G. J., Grill, E. & Schroeder, J. I. (2000) Nature 406, 731-734. [DOI] [PubMed] [Google Scholar]
- 41.Murata, Y., Pei, Z. M., Mori, I. C. & Schroeder, J. (2001) Plant Cell 13, 2513-2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stamler, J. S. & Meissner, G. (2001) Physiol. Rev. 81, 209-237. [DOI] [PubMed] [Google Scholar]