Abstract
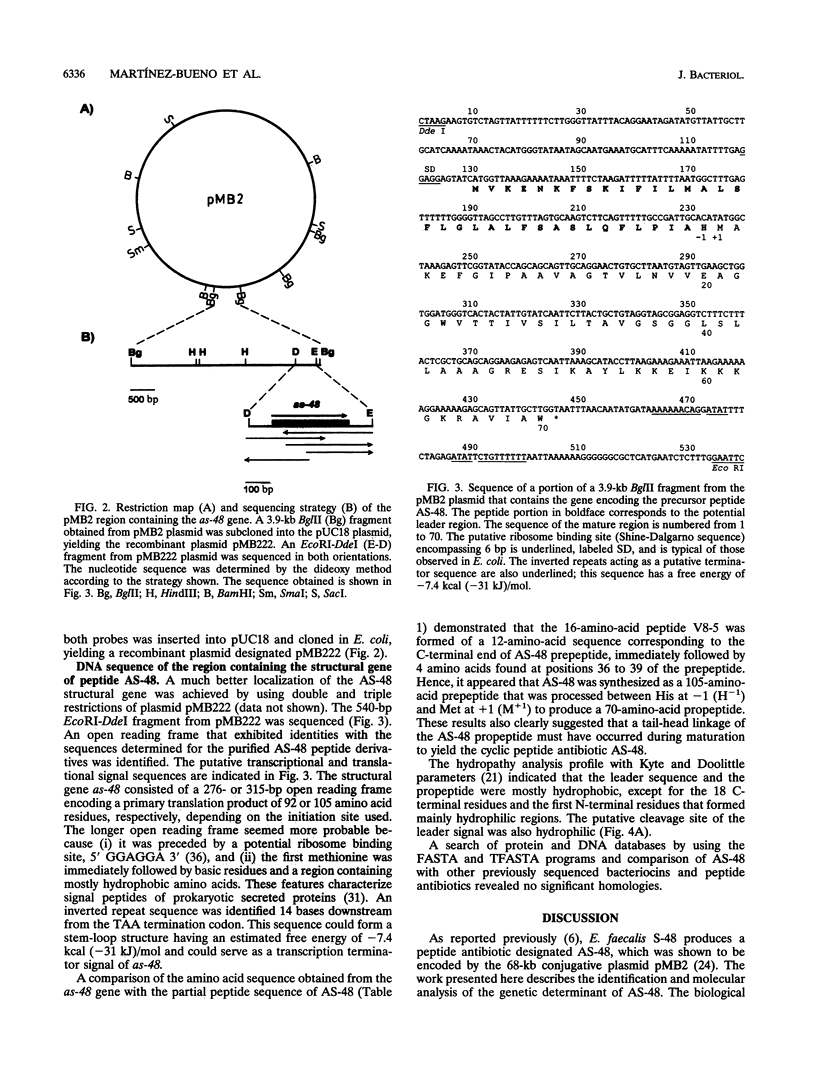
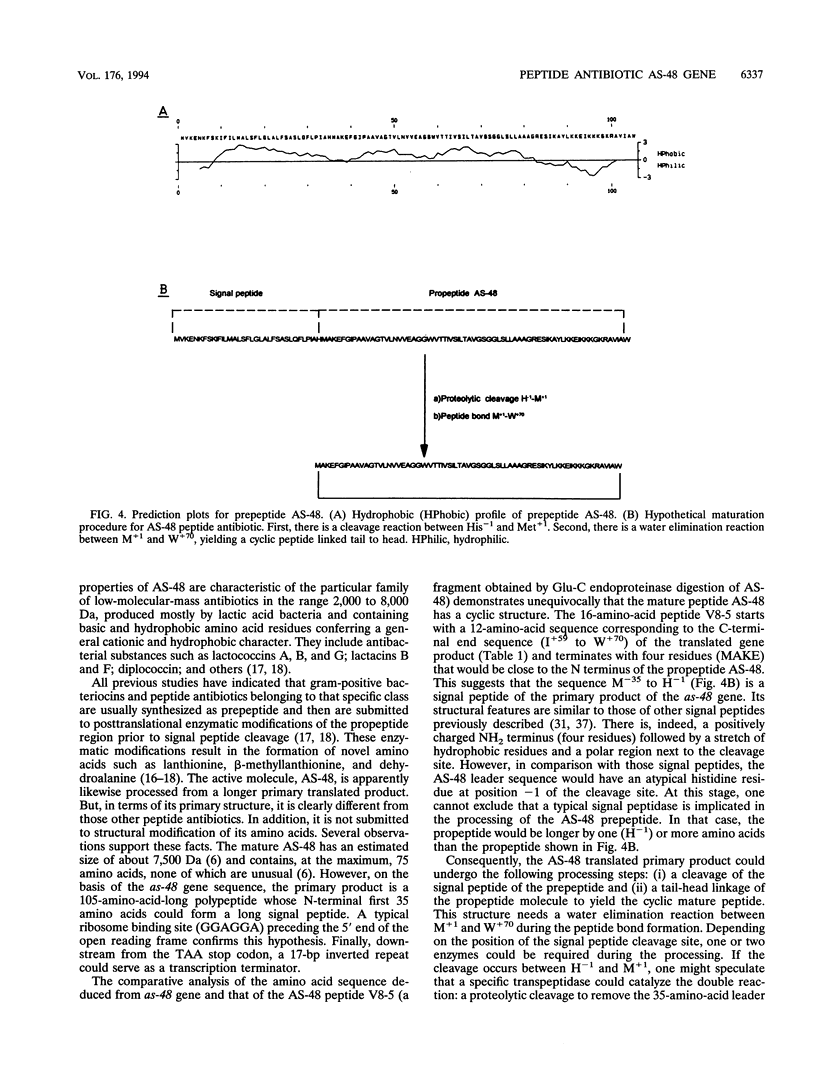
The structural gene of the enterococcal peptide antibiotic AS-48 (as-48) has been identified and cloned by using two degenerate 17-mer DNA oligonucleotides on the basis of the amino acid sequences of two peptides obtained by digestion of the antibiotic with Glu-C endoproteinase. That as-48 gene codes for a 105-amino-acid prepeptide, giving rise to a 70-amino-acid mature protein. Comparative analysis demonstrated that the 16-amino-acid sequence of one of the AS-48 Glu-C peptides, designated V8-5, was composed of a 12-amino-acid sequence corresponding to the C-terminal end sequence (from isoleucine +59 to tryptophan +70 [I+59 to W+70]) of the prepeptide and terminated in four residues forming the N terminus (M+1 to E+4) of a putative AS-48 propeptide. These data, combined with the characteristics of the gene sequence, strongly suggested that the antibiotic peptide was a 70-residue cyclic molecule. We propose that the AS-48 translated primary product is very likely submitted to a posttranslational modification during secretion (i) by an atypical or a typical signal peptidase that cleaves off a 35-residue or shorter signal peptide, respectively, from the prepeptide molecule and (ii) by the linkage of the methionine residue (M+1) to the C-terminal tryptophan residue (W+70) to obtain the cyclic peptide (a tail-head linkage).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., McKay L. L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983 Sep;46(3):549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman G. W., Banerjee S., Hansen J. N. Structure, expression, and evolution of a gene encoding the precursor of nisin, a small protein antibiotic. J Biol Chem. 1988 Nov 5;263(31):16260–16266. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Movable genetic elements and antibiotic resistance in enterococci. Eur J Clin Microbiol Infect Dis. 1990 Feb;9(2):90–102. doi: 10.1007/BF01963632. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez A., Valdivia E., Maqueda M., Montoya E. Production of bacteriocin-like substances by group D streptococci of human origin. Microbios. 1985;43(176S):223–232. [PubMed] [Google Scholar]
- Gao F. H., Abee T., Konings W. N. Mechanism of action of the peptide antibiotic nisin in liposomes and cytochrome c oxidase-containing proteoliposomes. Appl Environ Microbiol. 1991 Aug;57(8):2164–2170. doi: 10.1128/aem.57.8.2164-2170.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez A., Giménez-Gallego G., Maqueda M., Valdivia E. Purification and amino acid composition of peptide antibiotic AS-48 produced by Streptococcus (Enterococcus) faecalis subsp. liquefaciens S-48. Antimicrob Agents Chemother. 1989 Apr;33(4):437–441. doi: 10.1128/aac.33.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez A., Maqueda M., Martínez-Bueno M., Valdivia E. Bactericidal and bacteriolytic action of peptide antibiotic AS-48 against gram-positive and gram-negative bacteria and other organisms. Res Microbiol. 1989 Jan;140(1):57–68. doi: 10.1016/0923-2508(89)90060-0. [DOI] [PubMed] [Google Scholar]
- Gálvez A., Maqueda M., Martínez-Bueno M., Valdivia E. Permeation of bacterial cells, permeation of cytoplasmic and artificial membrane vesicles, and channel formation on lipid bilayers by peptide antibiotic AS-48. J Bacteriol. 1991 Jan;173(2):886–892. doi: 10.1128/jb.173.2.886-892.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holo H., Nilssen O., Nes I. F. Lactococcin A, a new bacteriocin from Lactococcus lactis subsp. cremoris: isolation and characterization of the protein and its gene. J Bacteriol. 1991 Jun;173(12):3879–3887. doi: 10.1128/jb.173.12.3879-3887.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ike Y., Flannagan S. E., Clewell D. B. Hyperhemolytic phenomena associated with insertions of Tn916 into the hemolysin determinant of Enterococcus faecalis plasmid pAD1. J Bacteriol. 1992 Mar;174(6):1801–1809. doi: 10.1128/jb.174.6.1801-1809.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ike Y., Hashimoto H., Clewell D. B. Hemolysin of Streptococcus faecalis subspecies zymogenes contributes to virulence in mice. Infect Immun. 1984 Aug;45(2):528–530. doi: 10.1128/iai.45.2.528-530.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev. 1993 Sep;12(1-3):39–85. doi: 10.1111/j.1574-6976.1993.tb00012.x. [DOI] [PubMed] [Google Scholar]
- Kolter R., Moreno F. Genetics of ribosomally synthesized peptide antibiotics. Annu Rev Microbiol. 1992;46:141–163. doi: 10.1146/annurev.mi.46.100192.001041. [DOI] [PubMed] [Google Scholar]
- Kordel M., Benz R., Sahl H. G. Mode of action of the staphylococcinlike peptide Pep 5: voltage-dependent depolarization of bacterial and artificial membranes. J Bacteriol. 1988 Jan;170(1):84–88. doi: 10.1128/jb.170.1.84-88.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer J. Inhibition of different serotypes of Listeria monocytogenes by enterocins in solid and liquid media. J Med Microbiol. 1977 Aug;10(3):367–372. doi: 10.1099/00222615-10-3-367. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- López-Lara I., Gálvez A., Martínez-Bueno M., Maqueda M., Valdivia E. Purification, characterization, and biological effects of a second bacteriocin from Enterococcus faecalis ssp. liquefaciens S-48 and its mutant strain B-48-28. Can J Microbiol. 1991 Oct;37(10):769–774. doi: 10.1139/m91-132. [DOI] [PubMed] [Google Scholar]
- Martínez-Bueno M., Gálvez A., Maqueda M., Valdivia E. Genetic stability of the antagonistic character of Enterococcus faecalis ssp. liquefaciens and the detection of a new inhibitory bacteriocin-like substance. Folia Microbiol (Praha) 1990;35(2):113–123. doi: 10.1007/BF02820767. [DOI] [PubMed] [Google Scholar]
- Martínez-Bueno M., Gálvez A., Valdivia E., Maqueda M. A transferable plasmid associated with AS-48 production in Enterococcus faecalis. J Bacteriol. 1990 May;172(5):2817–2818. doi: 10.1128/jb.172.5.2817-2818.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Bueno M., Valdivia E., Gálvez A., Maqueda M. Transfer of a plasmid determining bacteriocin Bc-48 production and immunity, and response to sexual pheromones in Enterococcus faecalis S-48. Plasmid. 1992 Jul;28(1):61–69. doi: 10.1016/0147-619x(92)90036-a. [DOI] [PubMed] [Google Scholar]
- Mørtvedt C. I., Nissen-Meyer J., Sletten K., Nes I. F. Purification and amino acid sequence of lactocin S, a bacteriocin produced by Lactobacillus sake L45. Appl Environ Microbiol. 1991 Jun;57(6):1829–1834. doi: 10.1128/aem.57.6.1829-1834.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen-Meyer J., Holo H., Håvarstein L. S., Sletten K., Nes I. F. A novel lactococcal bacteriocin whose activity depends on the complementary action of two peptides. J Bacteriol. 1992 Sep;174(17):5686–5692. doi: 10.1128/jb.174.17.5686-5692.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojcius D. M., Young J. D. Cytolytic pore-forming proteins and peptides: is there a common structural motif? Trends Biochem Sci. 1991 Jun;16(6):225–229. doi: 10.1016/0968-0004(91)90090-i. [DOI] [PubMed] [Google Scholar]
- Pearson W. R. Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol. 1990;183:63–98. doi: 10.1016/0076-6879(90)83007-v. [DOI] [PubMed] [Google Scholar]
- Piard J. C., Kuipers O. P., Rollema H. S., Desmazeaud M. J., de Vos W. M. Structure, organization, and expression of the lct gene for lacticin 481, a novel lantibiotic produced by Lactococcus lactis. J Biol Chem. 1993 Aug 5;268(22):16361–16368. [PubMed] [Google Scholar]
- Pugsley A. P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993 Mar;57(1):50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell N., Entian K. D., Schneider U., Götz F., Zähner H., Kellner R., Jung G. Prepeptide sequence of epidermin, a ribosomally synthesized antibiotic with four sulphide-rings. Nature. 1988 May 19;333(6170):276–278. doi: 10.1038/333276a0. [DOI] [PubMed] [Google Scholar]
- Schüller F., Benz R., Sahl H. G. The peptide antibiotic subtilin acts by formation of voltage-dependent multi-state pores in bacterial and artificial membranes. Eur J Biochem. 1989 Jun 1;182(1):181–186. doi: 10.1111/j.1432-1033.1989.tb14815.x. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Simonen M., Palva I. Protein secretion in Bacillus species. Microbiol Rev. 1993 Mar;57(1):109–137. doi: 10.1128/mr.57.1.109-137.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Venema K., Abee T., Haandrikman A. J., Leenhouts K. J., Kok J., Konings W. N., Venema G. Mode of Action of Lactococcin B, a Thiol-Activated Bacteriocin from Lactococcus lactis. Appl Environ Microbiol. 1993 Apr;59(4):1041–1048. doi: 10.1128/aem.59.4.1041-1048.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi Y., Clewell D. B. Recombination-deficient mutant of Streptococcus faecalis. J Bacteriol. 1980 Aug;143(2):966–970. doi: 10.1128/jb.143.2.966-970.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]