Abstract
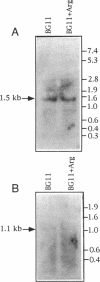
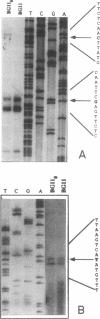
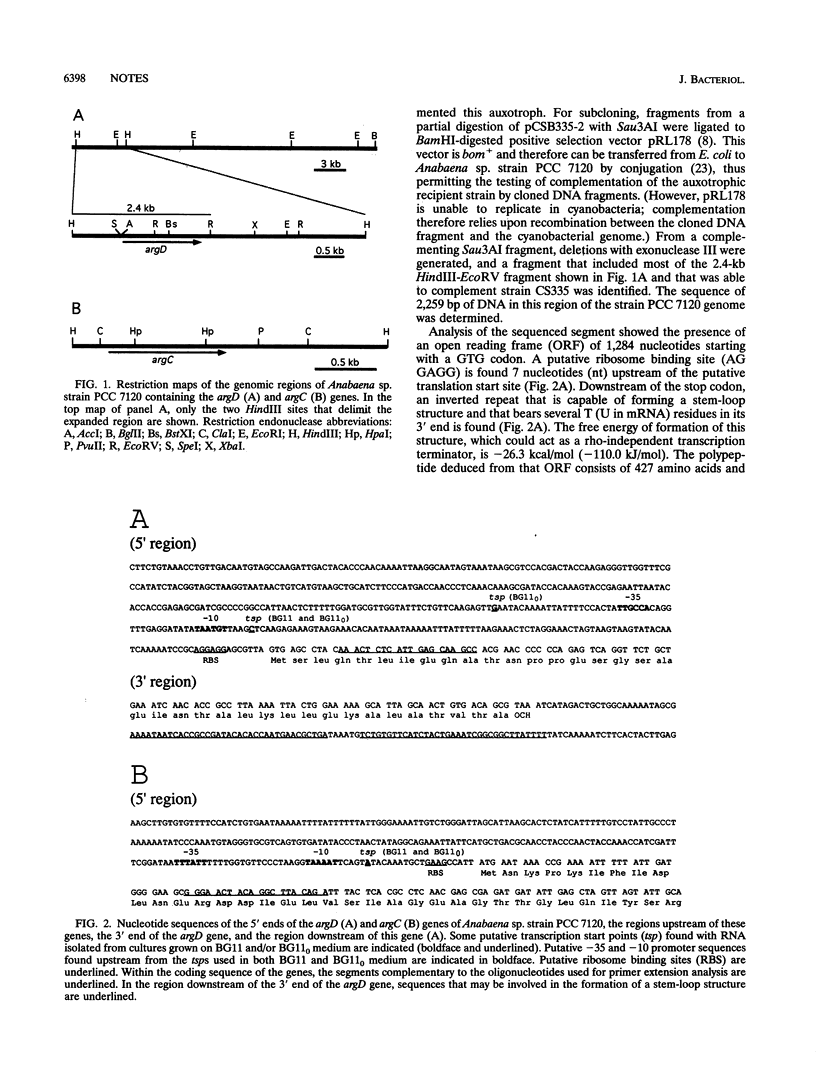
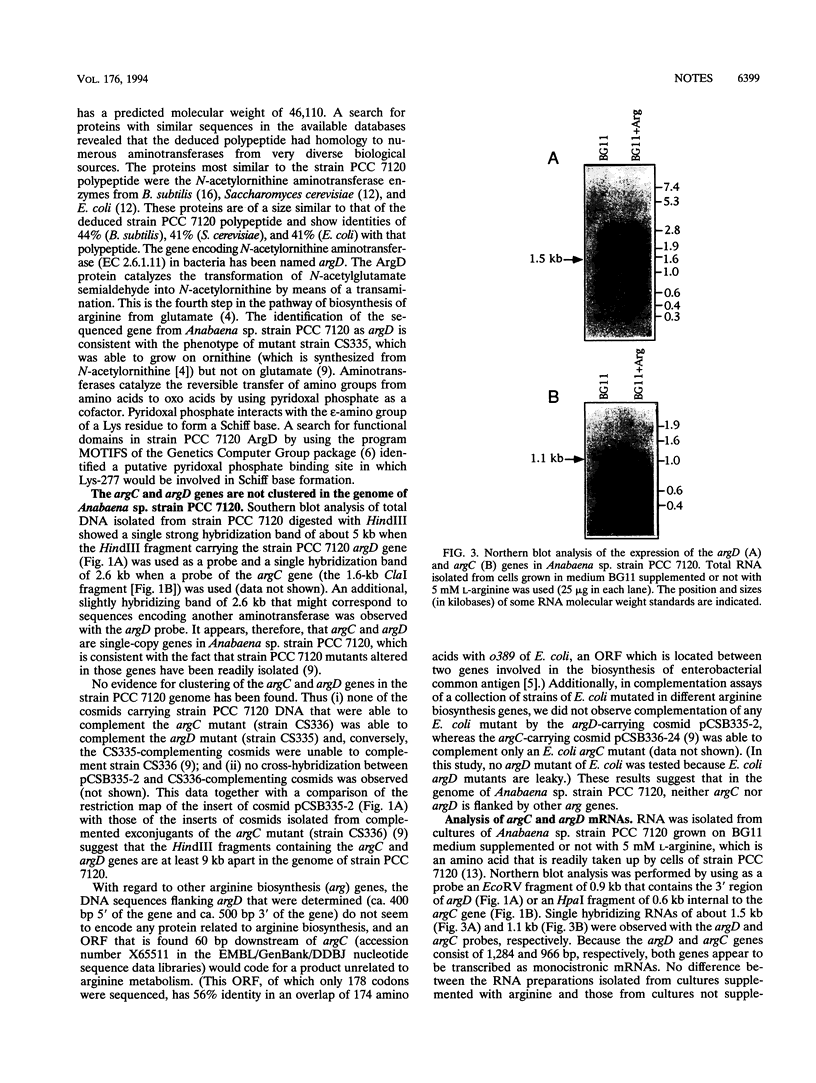
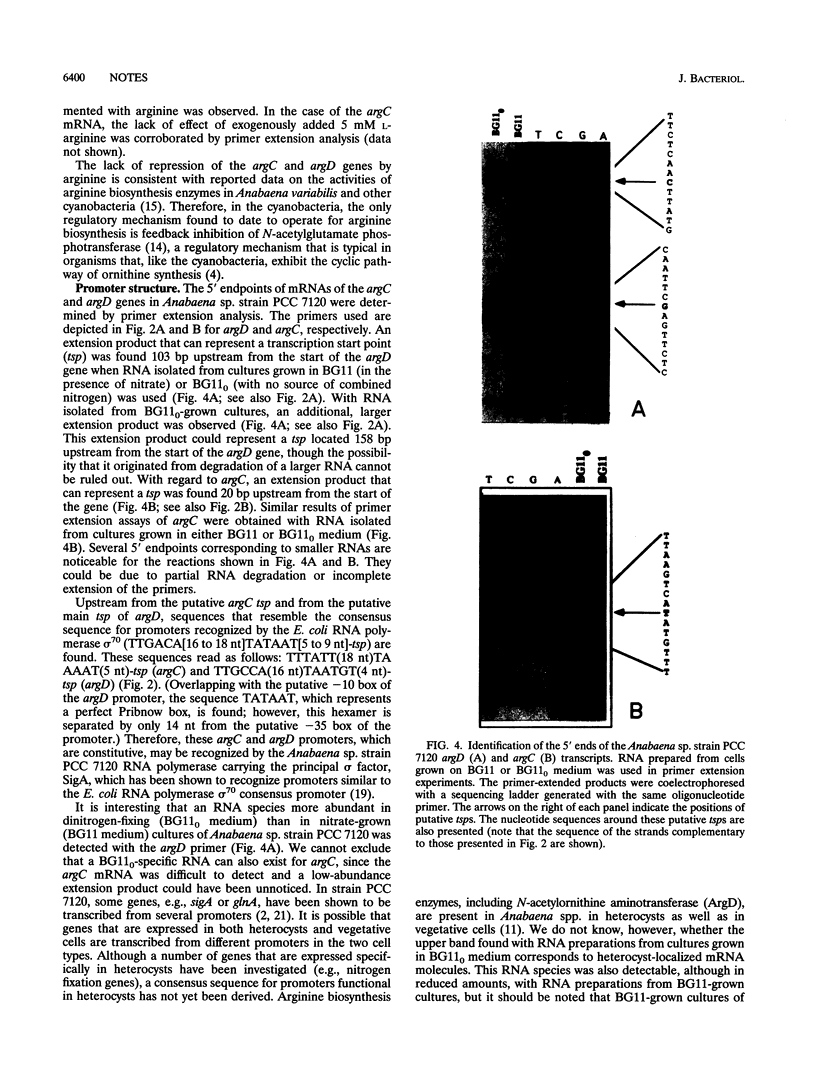
A cloned DNA fragment from Anabaena sp. strain PCC 7120 that complements an arginine auxotrophic mutant from the same organism was found to include an open reading frame encoding a 427-residue polypeptide that is homologous to N-acetylornithine aminotransferase from Bacillus subtilis, Escherichia coli, and Saccharomyces cerevisiae. The gene encoding N-acetylornithine aminotransferase in bacteria has been named argD. The expression of Anabaena sp. strain PCC 7120 argD, as well as of argC, was analyzed at the mRNA level. Both genes were transcribed as monocistronic mRNAs, and their expression was not affected by exogenously added arginine. Primer extension analysis identified transcription start points for both genes which were preceded by sequences similar to that of the E. coli RNA polymerase sigma 70 consensus promoter. A second transcription start point for the argD gene that is not preceded by a sigma 70 consensus promoter was detected in dinitrogen-grown cultures.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brahamsha B., Haselkorn R. Isolation and characterization of the gene encoding the principal sigma factor of the vegetative cell RNA polymerase from the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1991 Apr;173(8):2442–2450. doi: 10.1128/jb.173.8.2442-2450.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y. P., Wolk C. P. Use of a conditionally lethal gene in Anabaena sp. strain PCC 7120 to select for double recombinants and to entrap insertion sequences. J Bacteriol. 1990 Jun;172(6):3138–3145. doi: 10.1128/jb.172.6.3138-3145.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunin R., Glansdorff N., Piérard A., Stalon V. Biosynthesis and metabolism of arginine in bacteria. Microbiol Rev. 1986 Sep;50(3):314–352. doi: 10.1128/mr.50.3.314-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels D. L., Plunkett G., 3rd, Burland V., Blattner F. R. Analysis of the Escherichia coli genome: DNA sequence of the region from 84.5 to 86.5 minutes. Science. 1992 Aug 7;257(5071):771–778. doi: 10.1126/science.1379743. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhai J. Strong and regulated promoters in the cyanobacterium Anabaena PCC 7120. FEMS Microbiol Lett. 1993 Dec 1;114(2):179–184. doi: 10.1111/j.1574-6968.1993.tb06570.x. [DOI] [PubMed] [Google Scholar]
- Elhai J., Wolk C. P. A versatile class of positive-selection vectors based on the nonviability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene. 1988 Aug 15;68(1):119–138. doi: 10.1016/0378-1119(88)90605-1. [DOI] [PubMed] [Google Scholar]
- Floriano B., Herrero A., Flores E. Isolation of arginine auxotrophs, cloning by mutant complementation, and sequence analysis of the argC gene from the cyanobacterium Anabaena species PCC 7120. Mol Microbiol. 1992 Aug;6(15):2085–2094. doi: 10.1111/j.1365-2958.1992.tb01381.x. [DOI] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Heimberg H., Boyen A., Crabeel M., Glansdorff N. Escherichia coli and Saccharomyces cerevisiae acetylornithine aminotransferase: evolutionary relationship with ornithine aminotransferase. Gene. 1990 May 31;90(1):69–78. doi: 10.1016/0378-1119(90)90440-3. [DOI] [PubMed] [Google Scholar]
- Herrero A., Flores E. Transport of basic amino acids by the dinitrogen-fixing cyanobacterium Anabaena PCC 7120. J Biol Chem. 1990 Mar 5;265(7):3931–3935. [PubMed] [Google Scholar]
- Hoare D. S., Hoare S. L. Feedback regulation of arginine biosynthesis in blue-green algae and photosynthetic bacteria. J Bacteriol. 1966 Aug;92(2):375–379. doi: 10.1128/jb.92.2.375-379.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood W., Carr N. G. Apparent lack of control by repression of arginine metabolism in blue-green algae. J Bacteriol. 1971 Jul;107(1):365–367. doi: 10.1128/jb.107.1.365-367.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider G. J., Lang J. D., Haselkorn R. Promoter recognition by the RNA polymerase from vegetative cells of the cyanobacterium Anabaena 7120. Gene. 1991 Aug 30;105(1):51–60. doi: 10.1016/0378-1119(91)90513-b. [DOI] [PubMed] [Google Scholar]
- Schneider G. J., Tumer N. E., Richaud C., Borbely G., Haselkorn R. Purification and characterization of RNA polymerase from the cyanobacterium Anabaena 7120. J Biol Chem. 1987 Oct 25;262(30):14633–14639. [PubMed] [Google Scholar]
- Wolk C. P., Vonshak A., Kehoe P., Elhai J. Construction of shuttle vectors capable of conjugative transfer from Escherichia coli to nitrogen-fixing filamentous cyanobacteria. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1561–1565. doi: 10.1073/pnas.81.5.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]