Abstract
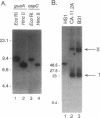
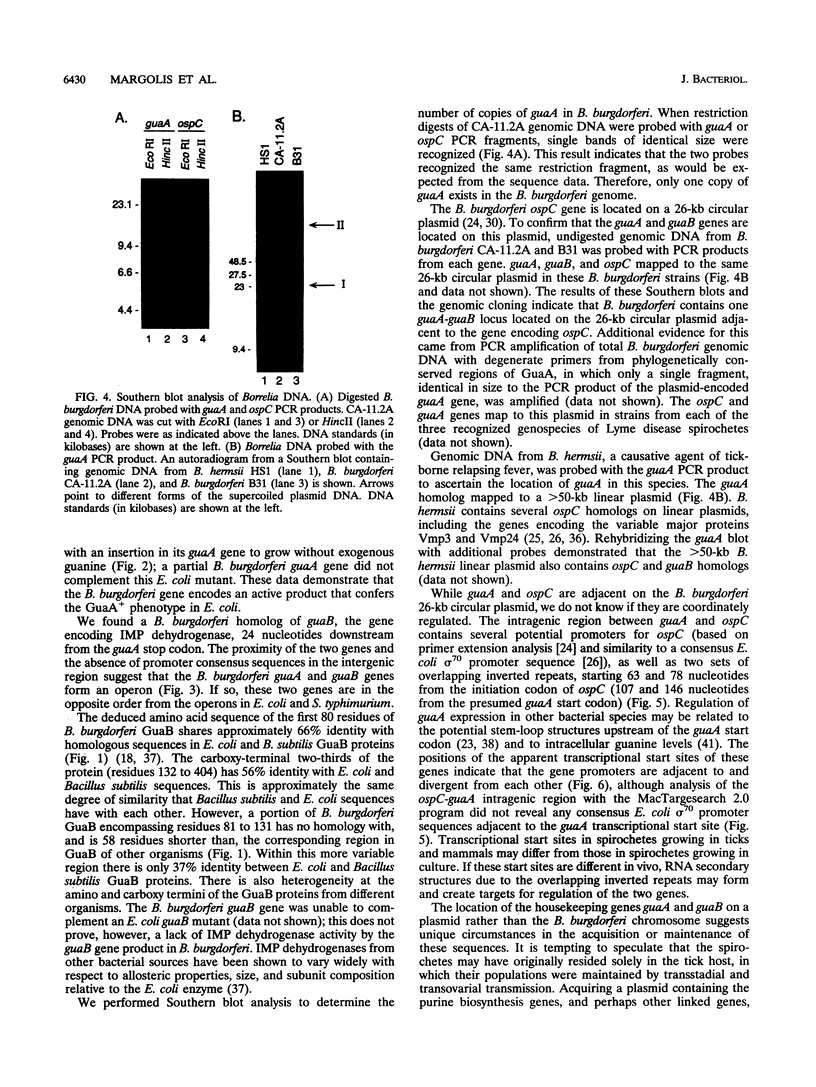
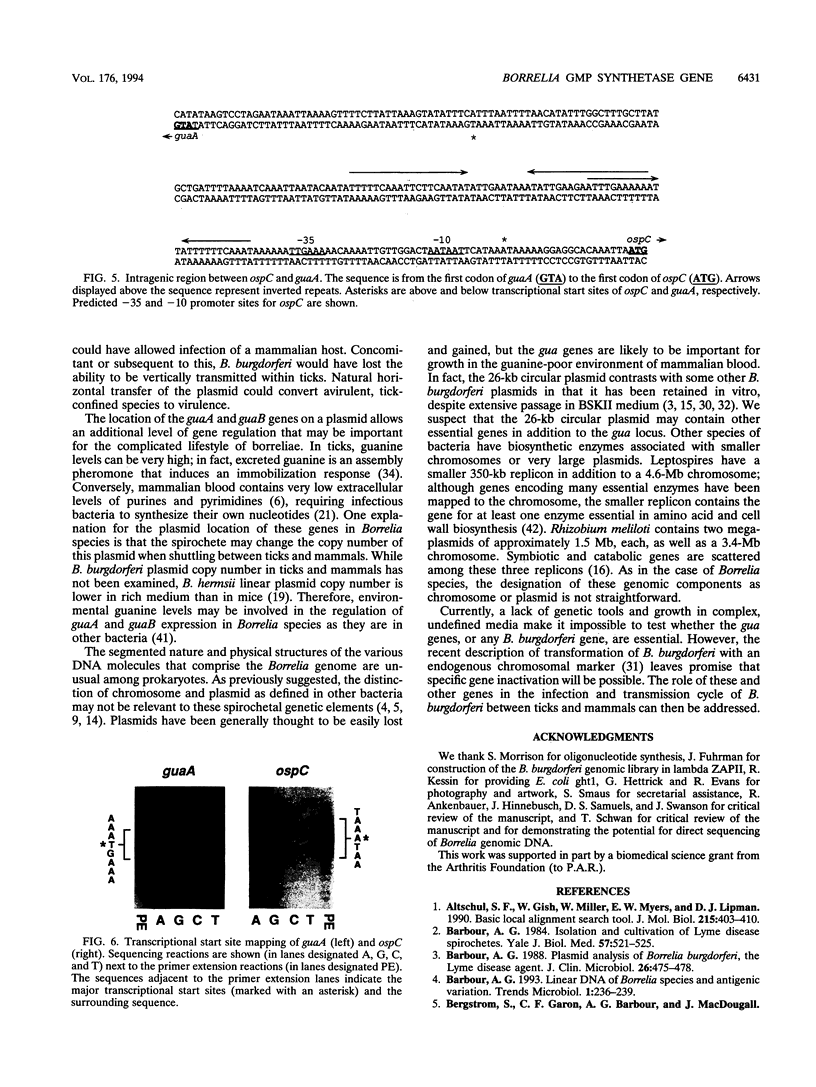
The Lyme disease spirochete Borrelia burgdorferi must survive in both its tick vector and its mammalian host to be maintained in nature. We have identified the B. burgdorferi guaA gene encoding GMP synthetase, an enzyme involved in de novo purine biosynthesis that is important for the survival of bacteria in mammalian blood. This gene encodes a functional product that will complement an Escherichia coli GMP synthetase mutant. The gene is located on a 26-kb circular plasmid, adjacent to and divergent from the gene encoding the outer surface protein C (OspC). The guaB gene homolog encoding IMP dehydrogenase, another enzyme in the purine biosynthetic pathway, is adjacent to guaA. In Borrelia hermsii, a tick-borne relapsing fever spirochete, the guaA and guaB genes are located on a linear plasmid. These are the first genes encoding proteins of known function to be mapped to a borrelial plasmid and the only example of genes encoding enzymes involved in the de novo purine biosynthesis pathway to be mapped to a plasmid in any organism. The unique plasmid location of these and perhaps other housekeeping genes may be a consequence of the segmented genomes in borreliae and reflect the need to adapt to both the arthropod and mammalian environments.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- BISHOP C., RANKINE D. M., TALBOTT J. H. The nucleotides in normal human blood. J Biol Chem. 1959 May;234(5):1233–1237. [PubMed] [Google Scholar]
- BURROWS T. W., BACON G. A. The basis of virulence in Pasteurella pestis: comparative behaviour of virulent and avirulent strains in vivo. Br J Exp Pathol. 1954 Apr;35(2):134–143. [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G. Linear DNA of Borrelia species and antigenic variation. Trends Microbiol. 1993 Sep;1(6):236–239. doi: 10.1016/0966-842x(93)90139-i. [DOI] [PubMed] [Google Scholar]
- Barbour A. G. Plasmid analysis of Borrelia burgdorferi, the Lyme disease agent. J Clin Microbiol. 1988 Mar;26(3):475–478. doi: 10.1128/jcm.26.3.475-478.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler F., Whalen J. A., Reinhardt B. N., Steere A. C. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis. 1993 Feb;167(2):392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- FURNESS G., ROWLEY D. Transduction of virulence within the species Salmonella typhimurium. J Gen Microbiol. 1956 Aug;15(1):140–145. doi: 10.1099/00221287-15-1-140. [DOI] [PubMed] [Google Scholar]
- Ferdows M. S., Barbour A. G. Megabase-sized linear DNA in the bacterium Borrelia burgdorferi, the Lyme disease agent. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5969–5973. doi: 10.1073/pnas.86.15.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikrig E., Telford S. R., 3rd, Barthold S. W., Kantor F. S., Spielman A., Flavell R. A. Elimination of Borrelia burgdorferi from vector ticks feeding on OspA-immunized mice. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5418–5421. doi: 10.1073/pnas.89.12.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie A. E., Crosa J. H. Co-operative autoregulation of a replication protein gene. Mol Microbiol. 1991 Dec;5(12):3015–3023. doi: 10.1111/j.1365-2958.1991.tb01861.x. [DOI] [PubMed] [Google Scholar]
- Goodrich J. A., Schwartz M. L., McClure W. R. Searching for and predicting the activity of sites for DNA binding proteins: compilation and analysis of the binding sites for Escherichia coli integration host factor (IHF). Nucleic Acids Res. 1990 Sep 11;18(17):4993–5000. doi: 10.1093/nar/18.17.4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes L. J., Wright D. J., Archard L. C. Segmented arrangement of Borrelia duttonii DNA and location of variant surface antigen genes. J Gen Microbiol. 1988 Jul;134(7):1785–1793. doi: 10.1099/00221287-134-7-1785. [DOI] [PubMed] [Google Scholar]
- Hinnebusch J., Barbour A. G. Linear- and circular-plasmid copy numbers in Borrelia burgdorferi. J Bacteriol. 1992 Aug;174(16):5251–5257. doi: 10.1128/jb.174.16.5251-5257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt R. J., McClelland M., Sobral B. W. Physical map of the genome of Rhizobium meliloti 1021. J Bacteriol. 1993 Nov;175(21):6945–6952. doi: 10.1128/jb.175.21.6945-6952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivánovics G., Marjai E., Dobozy A. The growth of purine mutants of Bacillus anthracis in the body of the mouse. J Gen Microbiol. 1968 Sep;53(2):147–162. doi: 10.1099/00221287-53-2-147. [DOI] [PubMed] [Google Scholar]
- Kitten T., Barbour A. G. The relapsing fever agent Borrelia hermsii has multiple copies of its chromosome and linear plasmids. Genetics. 1992 Oct;132(2):311–324. doi: 10.1093/genetics/132.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE H. B., MAURER R. L. Immunization with an induced avirulent auxotrophic mutant of Pseudomonas pseudomallei. J Immunol. 1958 Nov;81(5):433–438. [PubMed] [Google Scholar]
- Mahan M. J., Slauch J. M., Mekalanos J. J. Selection of bacterial virulence genes that are specifically induced in host tissues. Science. 1993 Jan 29;259(5095):686–688. doi: 10.1126/science.8430319. [DOI] [PubMed] [Google Scholar]
- Marconi R. T., Samuels D. S., Garon C. F. Transcriptional analyses and mapping of the ospC gene in Lyme disease spirochetes. J Bacteriol. 1993 Feb;175(4):926–932. doi: 10.1128/jb.175.4.926-932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi R. T., Samuels D. S., Schwan T. G., Garon C. F. Identification of a protein in several Borrelia species which is related to OspC of the Lyme disease spirochetes. J Clin Microbiol. 1993 Oct;31(10):2577–2583. doi: 10.1128/jcm.31.10.2577-2583.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis N., Hogan D., Cieplak W., Jr, Schwan T. G., Rosa P. A. Homology between Borrelia burgdorferi OspC and members of the family of Borrelia hermsii variable major proteins. Gene. 1994 May 27;143(1):105–110. doi: 10.1016/0378-1119(94)90613-0. [DOI] [PubMed] [Google Scholar]
- Margolis N., Rosa P. A. Regulation of expression of major outer surface proteins in Borrelia burgdorferi. Infect Immun. 1993 May;61(5):2207–2210. doi: 10.1128/iai.61.5.2207-2210.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadziene A., Wilske B., Ferdows M. S., Barbour A. G. The cryptic ospC gene of Borrelia burgdorferi B31 is located on a circular plasmid. Infect Immun. 1993 May;61(5):2192–2195. doi: 10.1128/iai.61.5.2192-2195.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels D. S., Mach K. E., Garon C. F. Genetic transformation of the Lyme disease agent Borrelia burgdorferi with coumarin-resistant gyrB. J Bacteriol. 1994 Oct;176(19):6045–6049. doi: 10.1128/jb.176.19.6045-6049.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W., Garon C. F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988 Aug;56(8):1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T. G., Schrumpf M. E., Karstens R. H., Clover J. R., Wong J., Daugherty M., Struthers M., Rosa P. A. Distribution and molecular analysis of Lyme disease spirochetes, Borrelia burgdorferi, isolated from ticks throughout California. J Clin Microbiol. 1993 Dec;31(12):3096–3108. doi: 10.1128/jcm.31.12.3096-3108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Harmon P. A. Growth in mouse peritoneal macrophages of Yersinia pestis lacking established virulence determinants. Infect Immun. 1984 Sep;45(3):649–654. doi: 10.1128/iai.45.3.649-654.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen M., Frederiksen B., Lebech A. M., Vuust J., Hansen K. Polymorphism in ospC gene of Borrelia burgdorferi and immunoreactivity of OspC protein: implications for taxonomy and for use of OspC protein as a diagnostic antigen. J Clin Microbiol. 1993 Oct;31(10):2570–2576. doi: 10.1128/jcm.31.10.2570-2576.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedeman A. A., Smith J. M. Nucleotide sequence of the guaB locus encoding IMP dehydrogenase of Escherichia coli K12. Nucleic Acids Res. 1985 Feb 25;13(4):1303–1316. doi: 10.1093/nar/13.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedeman A. A., Smith J. M., Zalkin H. Nucleotide sequence of the guaA gene encoding GMP synthetase of Escherichia coli K12. J Biol Chem. 1985 Jul 25;260(15):8676–8679. [PubMed] [Google Scholar]
- Van Lookeren Campagne M. M., Franke J., Kessin R. H. Functional cloning of a Dictyostelium discoideum cDNA encoding GMP synthetase. J Biol Chem. 1991 Sep 5;266(25):16448–16452. [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Schierz G., Busch K. V. Immunochemical and immunological analysis of European Borrelia burgdorferi strains. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Dec;263(1-2):92–102. doi: 10.1016/s0176-6724(86)80108-0. [DOI] [PubMed] [Google Scholar]
- Zalkin H., Dixon J. E. De novo purine nucleotide biosynthesis. Prog Nucleic Acid Res Mol Biol. 1992;42:259–287. doi: 10.1016/s0079-6603(08)60578-4. [DOI] [PubMed] [Google Scholar]
- Zuerner R. L., Herrmann J. L., Saint Girons I. Comparison of genetic maps for two Leptospira interrogans serovars provides evidence for two chromosomes and intraspecies heterogeneity. J Bacteriol. 1993 Sep;175(17):5445–5451. doi: 10.1128/jb.175.17.5445-5451.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]