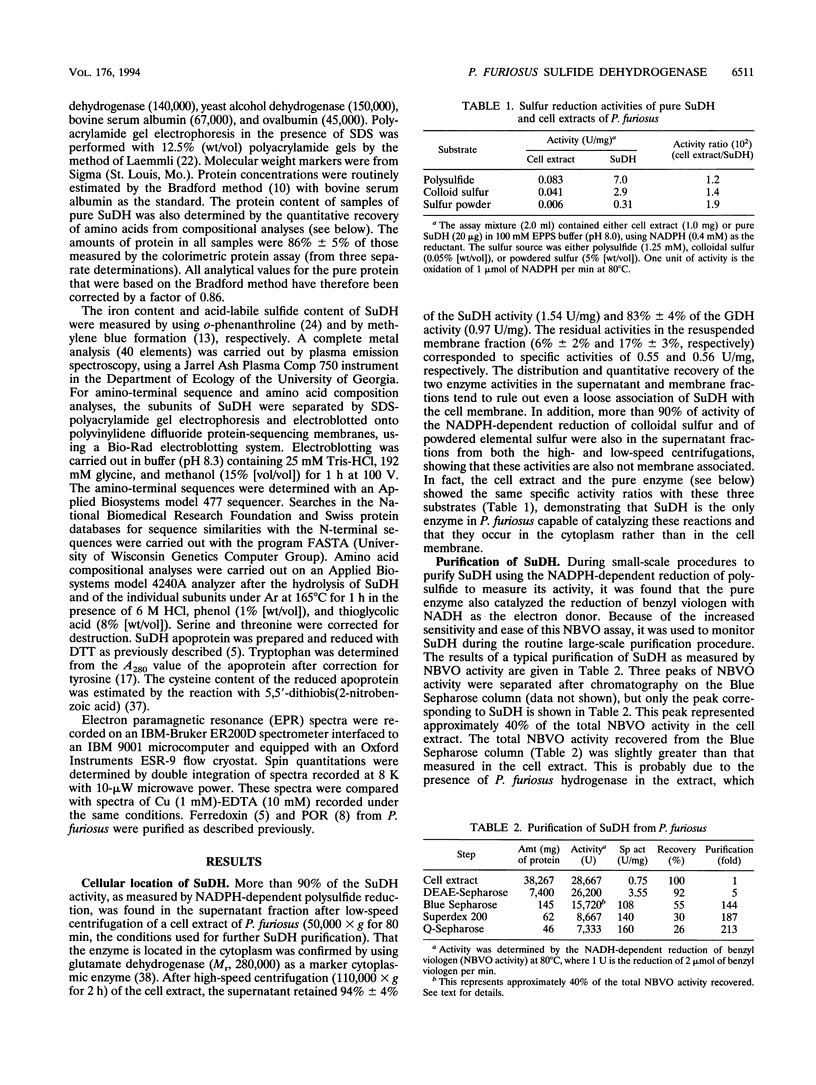
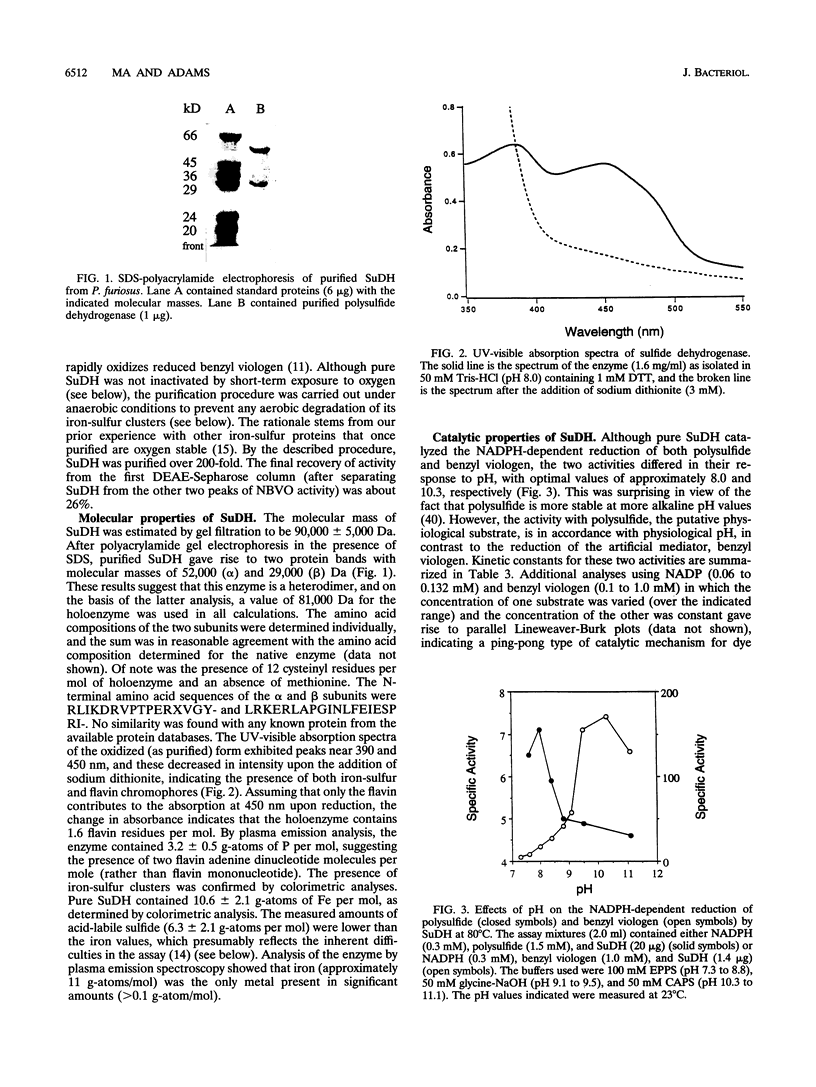
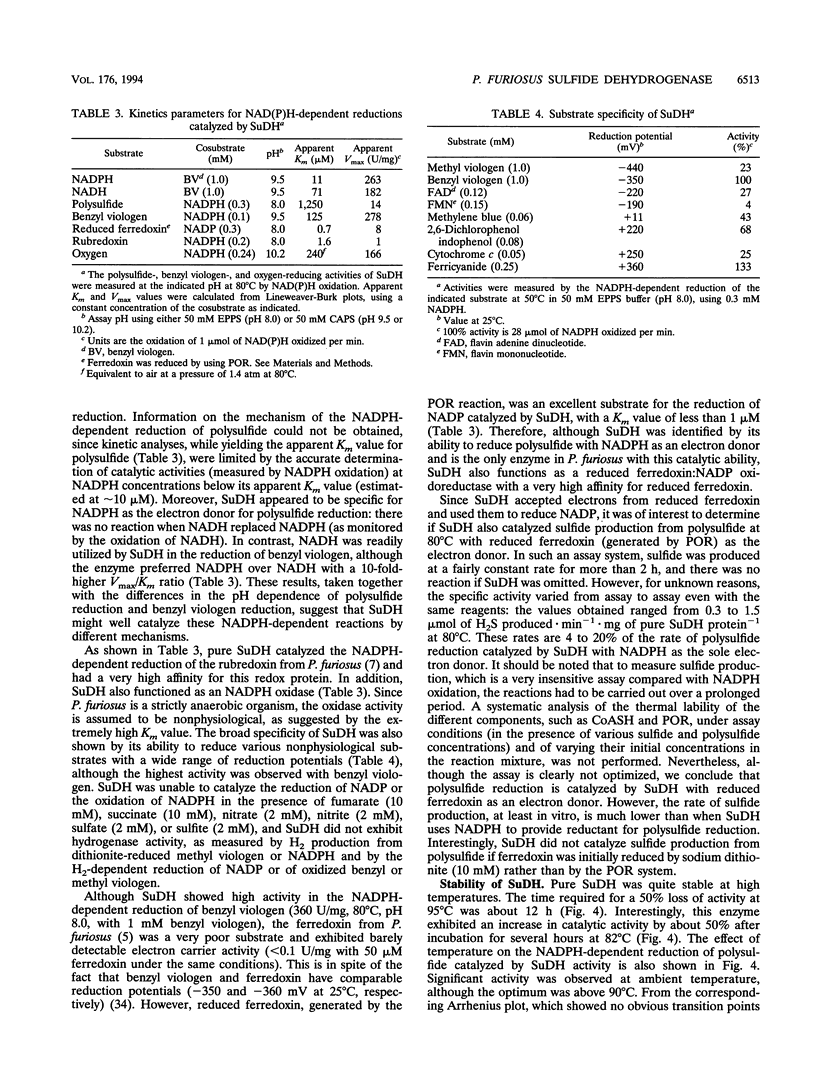
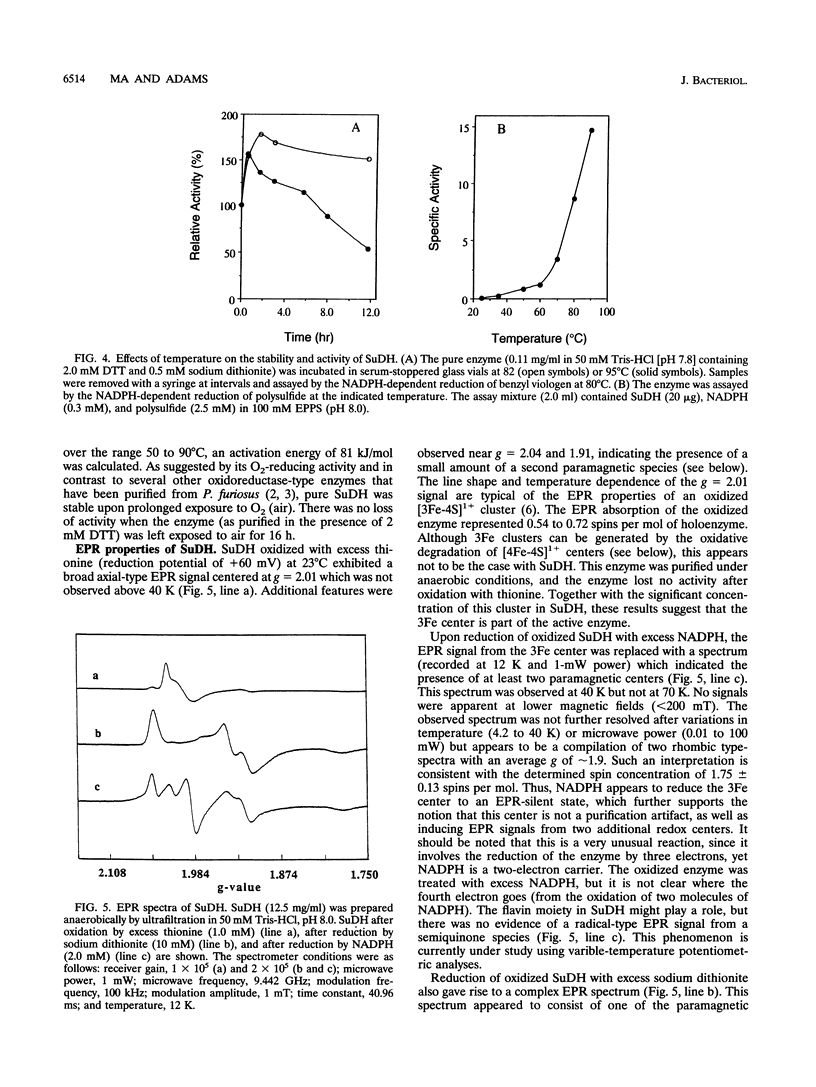
Abstract
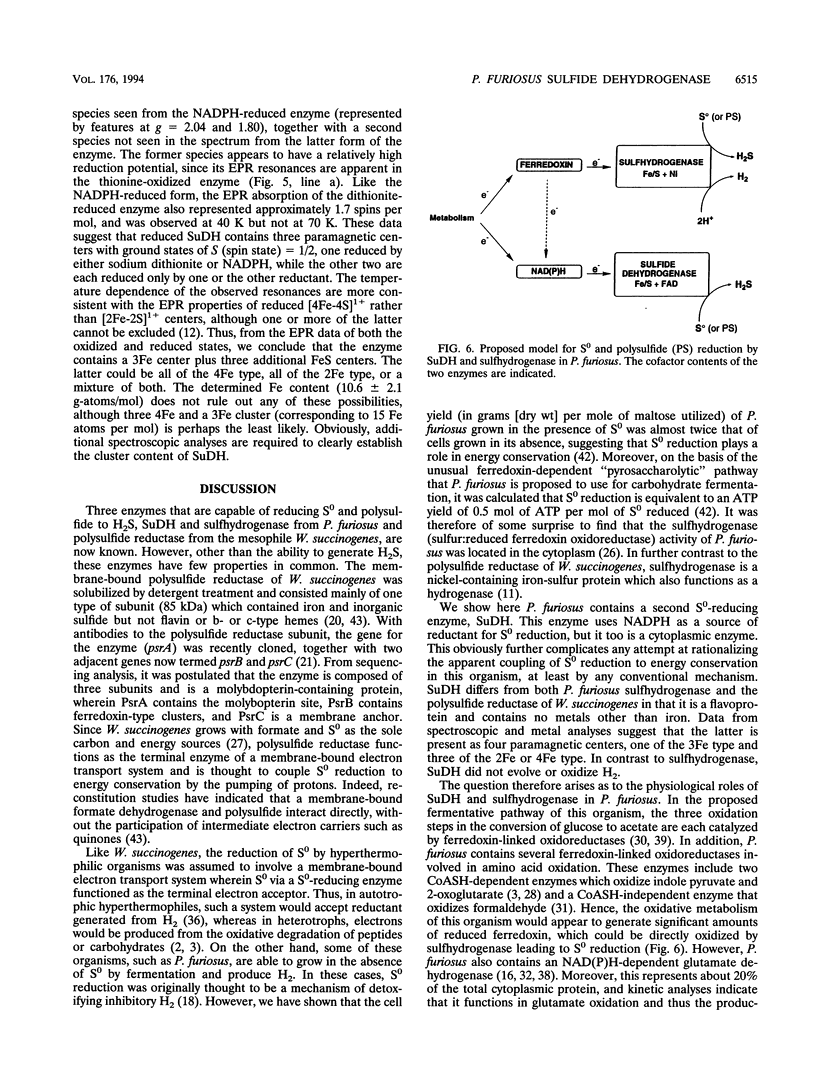
Pyrococcus furiosus is an anaerobic archaeon that grows optimally at 100 degrees C by the fermentation of carbohydrates yielding acetate, CO2, and H2 as the primary products. If elemental sulfur (S0) or polysulfide is added to the growth medium, H2S is also produced. The cytoplasmic hydrogenase of P. furiosus, which is responsible for H2 production with ferredoxin as the electron donor, has been shown to also catalyze the reduction of polysulfide to H2S (K. Ma, R. N. Schicho, R. M. Kelly, and M. W. W. Adams, Proc. Natl. Acad. Sci. USA 90:5341-5344, 1993). From the cytoplasm of this organism, we have now purified an enzyme, sulfide dehydrogenase (SuDH), which catalyzes the reduction of polysulfide to H2S with NADPH as the electron donor. SuDH is a heterodimer with subunits of 52,000 and 29,000 Da. SuDH contains flavin and approximately 11 iron and 6 acid-labile sulfide atoms per mol, but no other metals were detected. Analysis of the enzyme by electron paramagnetic resonance spectroscopy indicated the presence of four iron-sulfur centers, one of which was specifically reduced by NADPH. SuDH has a half-life at 95 degrees C of about 12 h and shows a 50% increase in activity after 12 h at 82 degrees C. The pure enzyme has a specific activity of 7 mumol of H2S produced.min-1.mg of protein-1 at 80 degrees C with polysulfide (1.2 mM) and NADPH (0.4 mM) as substrates. The apparent Km values were 1.25 mM and 11 microM, respectively. NADH was not utilized as an electron donor for polysulfide reduction. P. furiosus rubredoxin (K(m) = 1.6 microM) also functioned as an electron acceptor for SuDH, and SuDH catalyzed the reduction of NADP with reduced P. furiosus ferredoxin (K(m) = 0.7 microM) as an electron donor. The multiple activities of SuDH and its proposed role in the metabolism of S(o) and polysulfide are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. W. Enzymes and proteins from organisms that grow near and above 100 degrees C. Annu Rev Microbiol. 1993;47:627–658. doi: 10.1146/annurev.mi.47.100193.003211. [DOI] [PubMed] [Google Scholar]
- Andreotti G., Cubellis M. V., Nitti G., Sannia G., Mai X., Marino G., Adams M. W. Characterization of aromatic aminotransferases from the hyperthermophilic archaeon Thermococcus litoralis. Eur J Biochem. 1994 Mar 1;220(2):543–549. doi: 10.1111/j.1432-1033.1994.tb18654.x. [DOI] [PubMed] [Google Scholar]
- Aono S., Bryant F. O., Adams M. W. A novel and remarkably thermostable ferredoxin from the hyperthermophilic archaebacterium Pyrococcus furiosus. J Bacteriol. 1989 Jun;171(6):3433–3439. doi: 10.1128/jb.171.6.3433-3439.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinert H., Thomson A. J. Three-iron clusters in iron-sulfur proteins. Arch Biochem Biophys. 1983 Apr 15;222(2):333–361. doi: 10.1016/0003-9861(83)90531-3. [DOI] [PubMed] [Google Scholar]
- Blake P. R., Park J. B., Bryant F. O., Aono S., Magnuson J. K., Eccleston E., Howard J. B., Summers M. F., Adams M. W. Determinants of protein hyperthermostability: purification and amino acid sequence of rubredoxin from the hyperthermophilic archaebacterium Pyrococcus furiosus and secondary structure of the zinc adduct by NMR. Biochemistry. 1991 Nov 12;30(45):10885–10895. doi: 10.1021/bi00109a012. [DOI] [PubMed] [Google Scholar]
- Blamey J. M., Adams M. W. Purification and characterization of pyruvate ferredoxin oxidoreductase from the hyperthermophilic archaeon Pyrococcus furiosus. Biochim Biophys Acta. 1993 Jan 15;1161(1):19–27. doi: 10.1016/0167-4838(93)90190-3. [DOI] [PubMed] [Google Scholar]
- Blumentals I. I., Itoh M., Olson G. J., Kelly R. M. Role of Polysulfides in Reduction of Elemental Sulfur by the Hyperthermophilic Archaebacterium Pyrococcus furiosus. Appl Environ Microbiol. 1990 May;56(5):1255–1262. doi: 10.1128/aem.56.5.1255-1262.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bryant F. O., Adams M. W. Characterization of hydrogenase from the hyperthermophilic archaebacterium, Pyrococcus furiosus. J Biol Chem. 1989 Mar 25;264(9):5070–5079. [PubMed] [Google Scholar]
- Chen J. S., Mortenson L. E. Inhibition of methylene blue formation during determination of the acid-labile sulfide of iron-sulfur protein samples containing dithionite. Anal Biochem. 1977 May 1;79(1-2):157–165. doi: 10.1016/0003-2697(77)90390-6. [DOI] [PubMed] [Google Scholar]
- Chen L., Liu M. Y., LeGall J., Fareleira P., Santos H., Xavier A. V. Rubredoxin oxidase, a new flavo-hemo-protein, is the site of oxygen reduction to water by the "strict anaerobe" Desulfovibrio gigas. Biochem Biophys Res Commun. 1993 May 28;193(1):100–105. doi: 10.1006/bbrc.1993.1595. [DOI] [PubMed] [Google Scholar]
- Conover R. C., Kowal A. T., Fu W. G., Park J. B., Aono S., Adams M. W., Johnson M. K. Spectroscopic characterization of the novel iron-sulfur cluster in Pyrococcus furiosus ferredoxin. J Biol Chem. 1990 May 25;265(15):8533–8541. [PubMed] [Google Scholar]
- Consalvi V., Chiaraluce R., Politi L., Vaccaro R., De Rosa M., Scandurra R. Extremely thermostable glutamate dehydrogenase from the hyperthermophilic archaebacterium Pyrococcus furiosus. Eur J Biochem. 1991 Dec 18;202(3):1189–1196. doi: 10.1111/j.1432-1033.1991.tb16489.x. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Krafft T., Bokranz M., Klimmek O., Schröder I., Fahrenholz F., Kojro E., Kröger A. Cloning and nucleotide sequence of the psrA gene of Wolinella succinogenes polysulphide reductase. Eur J Biochem. 1992 Jun 1;206(2):503–510. doi: 10.1111/j.1432-1033.1992.tb16953.x. [DOI] [PubMed] [Google Scholar]
- LOVENBERG W., BUCHANAN B. B., RABINOWITZ J. C. STUDIES ON THE CHEMICAL NATURE OF CLOSTRIDIAL FERREDOXIN. J Biol Chem. 1963 Dec;238:3899–3913. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Faou A., Rajagopal B. S., Daniels L., Fauque G. Thiosulfate, polythionates and elemental sulfur assimilation and reduction in the bacterial world. FEMS Microbiol Rev. 1990 Aug;6(4):351–381. doi: 10.1111/j.1574-6968.1990.tb04107.x. [DOI] [PubMed] [Google Scholar]
- Ma K., Robb F. T., Adams M. W. Purification and characterization of NADP-specific alcohol dehydrogenase and glutamate dehydrogenase from the hyperthermophilic archaeon Thermococcus litoralis. Appl Environ Microbiol. 1994 Feb;60(2):562–568. doi: 10.1128/aem.60.2.562-568.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K., Schicho R. N., Kelly R. M., Adams M. W. Hydrogenase of the hyperthermophile Pyrococcus furiosus is an elemental sulfur reductase or sulfhydrogenase: evidence for a sulfur-reducing hydrogenase ancestor. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5341–5344. doi: 10.1073/pnas.90.11.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai X., Adams M. W. Indolepyruvate ferredoxin oxidoreductase from the hyperthermophilic archaeon Pyrococcus furiosus. A new enzyme involved in peptide fermentation. J Biol Chem. 1994 Jun 17;269(24):16726–16732. [PubMed] [Google Scholar]
- Mukund S., Adams M. W. Characterization of a novel tungsten-containing formaldehyde ferredoxin oxidoreductase from the hyperthermophilic archaeon, Thermococcus litoralis. A role for tungsten in peptide catabolism. J Biol Chem. 1993 Jun 25;268(18):13592–13600. [PubMed] [Google Scholar]
- Mukund S., Adams M. W. The novel tungsten-iron-sulfur protein of the hyperthermophilic archaebacterium, Pyrococcus furiosus, is an aldehyde ferredoxin oxidoreductase. Evidence for its participation in a unique glycolytic pathway. J Biol Chem. 1991 Aug 5;266(22):14208–14216. [PubMed] [Google Scholar]
- Ohshima T., Nishida N. Purification and properties of extremely thermostable glutamate dehydrogenases from two hyperthermophilic archaebacteria, Pyrococcus woesei and Pyrococcus furiosus. Biosci Biotechnol Biochem. 1993 Jun;57(6):945–951. doi: 10.1271/bbb.57.945. [DOI] [PubMed] [Google Scholar]
- Olsen G. J., Woese C. R., Overbeek R. The winds of (evolutionary) change: breathing new life into microbiology. J Bacteriol. 1994 Jan;176(1):1–6. doi: 10.1128/jb.176.1.1-6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. B., Fan C. L., Hoffman B. M., Adams M. W. Potentiometric and electron nuclear double resonance properties of the two spin forms of the [4Fe-4S]+ cluster in the novel ferredoxin from the hyperthermophilic archaebacterium Pyrococcus furiosus. J Biol Chem. 1991 Oct 15;266(29):19351–19356. [PubMed] [Google Scholar]
- Pfennig N., Biebl H. Desulfuromonas acetoxidans gen. nov. and sp. nov., a new anaerobic, sulfur-reducing, acetate-oxidizing bacterium. Arch Microbiol. 1976 Oct 11;110(1):3–12. doi: 10.1007/BF00416962. [DOI] [PubMed] [Google Scholar]
- Pihl T. D., Black L. K., Schulman B. A., Maier R. J. Hydrogen-oxidizing electron transport components in the hyperthermophilic archaebacterium Pyrodictium brockii. J Bacteriol. 1992 Jan;174(1):137–143. doi: 10.1128/jb.174.1.137-143.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddles P. W., Blakeley R. L., Zerner B. Reassessment of Ellman's reagent. Methods Enzymol. 1983;91:49–60. doi: 10.1016/s0076-6879(83)91010-8. [DOI] [PubMed] [Google Scholar]
- Robb F. T., Park J. B., Adams M. W. Characterization of an extremely thermostable glutamate dehydrogenase: a key enzyme in the primary metabolism of the hyperthermophilic archaebacterium, Pyrococcus furiosus. Biochim Biophys Acta. 1992 Apr 17;1120(3):267–272. doi: 10.1016/0167-4838(92)90247-b. [DOI] [PubMed] [Google Scholar]
- Schicho R. N., Ma K., Adams M. W., Kelly R. M. Bioenergetics of sulfur reduction in the hyperthermophilic archaeon Pyrococcus furiosus. J Bacteriol. 1993 Mar;175(6):1823–1830. doi: 10.1128/jb.175.6.1823-1830.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zöphel A., Kennedy M. C., Beinert H., Kroneck P. M. Investigations on microbial sulfur respiration. Isolation, purification, and characterization of cellular components from Spirillum 5175. Eur J Biochem. 1991 Feb 14;195(3):849–856. doi: 10.1111/j.1432-1033.1991.tb15774.x. [DOI] [PubMed] [Google Scholar]



