Abstract
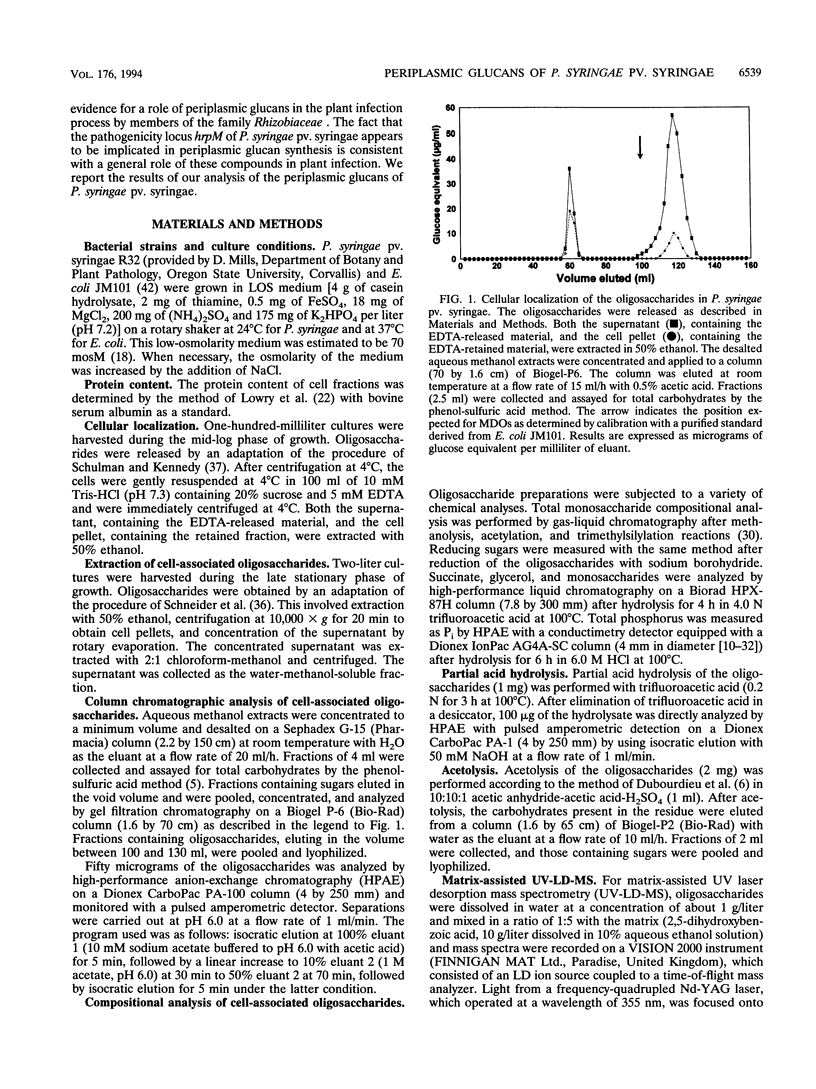
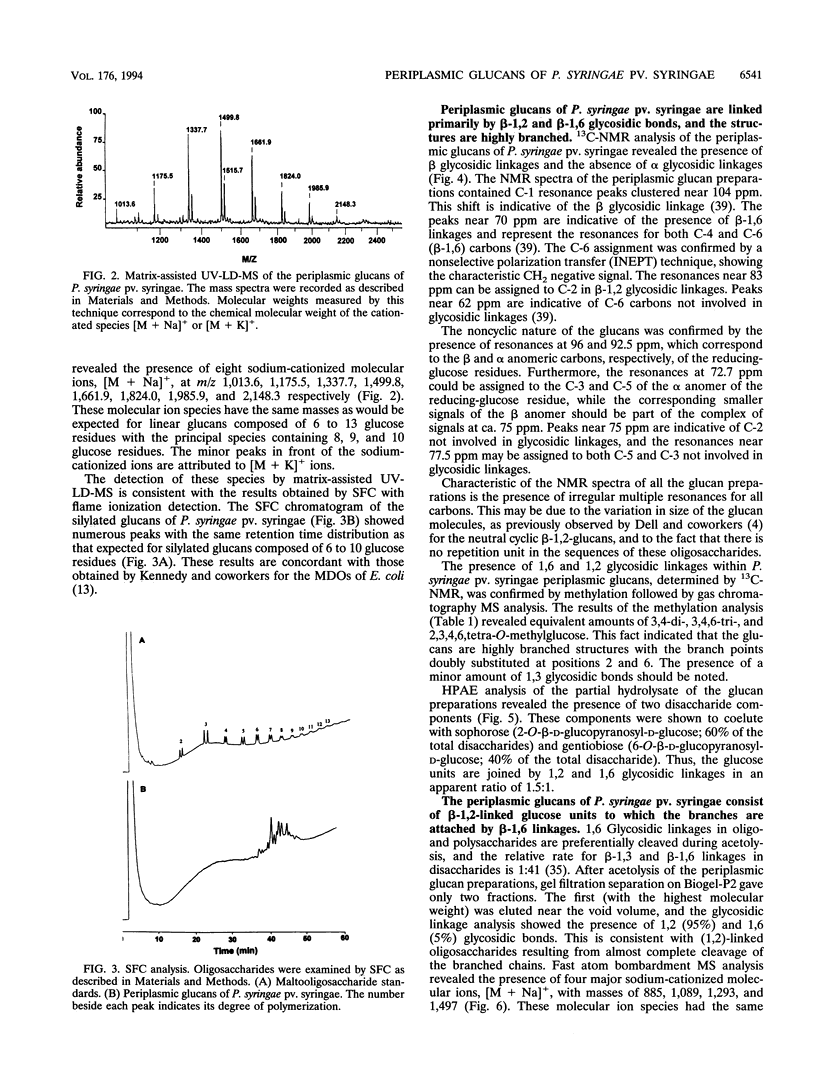
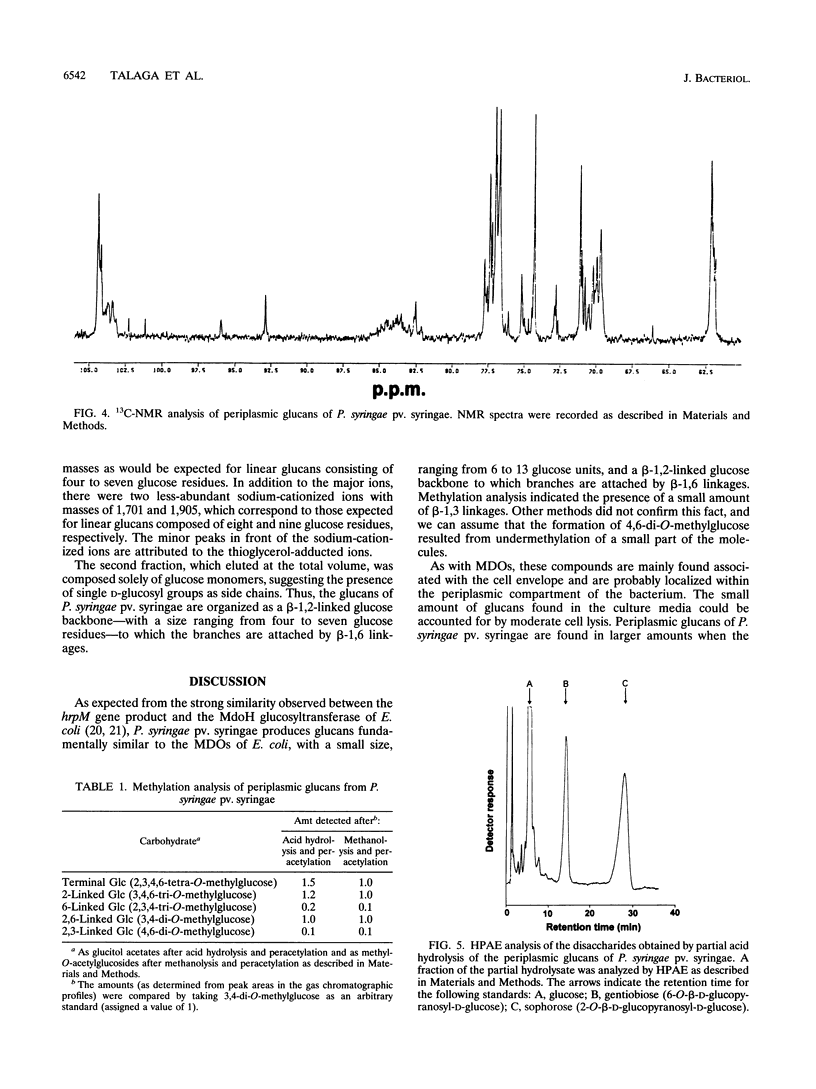
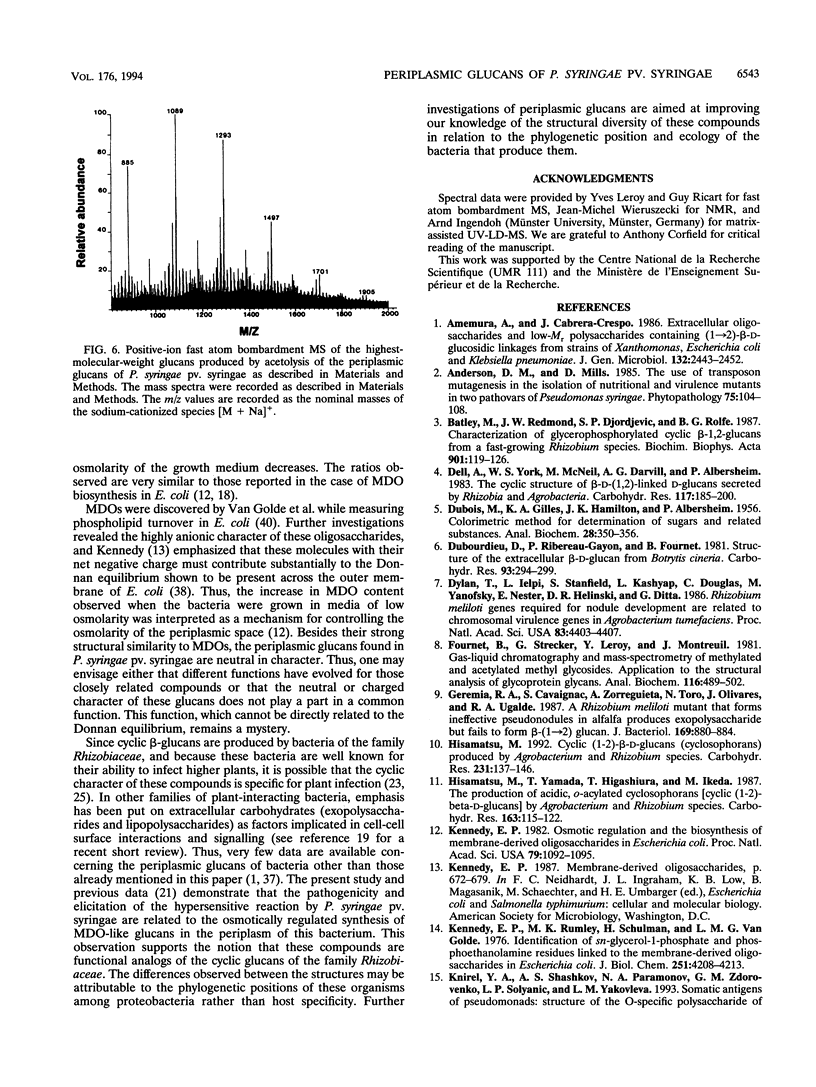
We report the initial characterization of glucans present in the periplasmic space of Pseudomonas syringae pv. syringae (strain R32). These compounds were found to be neutral, unsubstituted, and composed solely of glucose. Their size ranges from 6 to 13 glucose units/mol. Linkage studies and nuclear magnetic resonance analyses demonstrated that the glucans are linked by beta-1,2 and beta-1,6 glycosidic bonds. In contrast to the periplasmic glucans found in other plant pathogenic bacteria, the glucans of P. syringae pv. syringae are not cyclic but are highly branched structures. Acetolysis studies demonstrated that the backbone consists of beta-1,2-linked glucose units to which the branches are attached by beta-1,6 linkages. These periplasmic glucans were more abundant when the osmolarity of the growth medium was lower. Thus, P. syringae pv. syringae appears to synthesize periplasmic glucans in response to the osmolarity of the medium. The structural characteristics of these glucans are very similar to the membrane-derived oligosaccharides of Escherichia coli, apart from the neutral character, which contrasts with the highly anionic E. coli membrane-derived oligosaccharides.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amemura A., Cabrera-Crespo J. Extracellular oligosaccharides and low-Mr polysaccharides containing (1----2)-beta-D-glucosidic linkages from strains of Xanthomonas, Escherichia coli and Klebsiella pneumoniae. J Gen Microbiol. 1986 Sep;132(9):2443–2452. doi: 10.1099/00221287-132-9-2443. [DOI] [PubMed] [Google Scholar]
- Dylan T., Ielpi L., Stanfield S., Kashyap L., Douglas C., Yanofsky M., Nester E., Helinski D. R., Ditta G. Rhizobium meliloti genes required for nodule development are related to chromosomal virulence genes in Agrobacterium tumefaciens. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4403–4407. doi: 10.1073/pnas.83.12.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournet B., Strecker G., Leroy Y., Montreuil J. Gas--liquid chromatography and mass spectrometry of methylated and acetylated methyl glycosides. Application to the structural analysis of glycoprotein glycans. Anal Biochem. 1981 Sep 15;116(2):489–502. doi: 10.1016/0003-2697(81)90393-6. [DOI] [PubMed] [Google Scholar]
- Geremia R. A., Cavaignac S., Zorreguieta A., Toro N., Olivares J., Ugalde R. A. A Rhizobium meliloti mutant that forms ineffective pseudonodules in alfalfa produces exopolysaccharide but fails to form beta-(1----2) glucan. J Bacteriol. 1987 Feb;169(2):880–884. doi: 10.1128/jb.169.2.880-884.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisamatsu M. Cyclic (1----2)-beta-D-glucans (cyclosophorans) produced by Agrobacterium and Rhizobium species. Carbohydr Res. 1992 Jul 2;231:137–146. doi: 10.1016/0008-6215(92)84014-j. [DOI] [PubMed] [Google Scholar]
- Kennedy E. P. Osmotic regulation and the biosynthesis of membrane-derived oligosaccharides in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1092–1095. doi: 10.1073/pnas.79.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy E. P., Rumley M. K., Schulman H., Van Golde L. M. Identification of sn-glycero-1-phosphate and phosphoethanolamine residues linked to the membrane-derived Oligosaccharides of Escherichia coli. J Biol Chem. 1976 Jul 25;251(14):4208–4213. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacroix J. M., Loubens I., Tempête M., Menichi B., Bohin J. P. The mdoA locus of Escherichia coli consists of an operon under osmotic control. Mol Microbiol. 1991 Jul;5(7):1745–1753. doi: 10.1111/j.1365-2958.1991.tb01924.x. [DOI] [PubMed] [Google Scholar]
- Lacroix J. M., Tempête M., Menichi B., Bohin J. P. Molecular cloning and expression of a locus (mdoA) implicated in the biosynthesis of membrane-derived oligosaccharides in Escherichia coli. Mol Microbiol. 1989 Sep;3(9):1173–1182. doi: 10.1111/j.1365-2958.1989.tb00267.x. [DOI] [PubMed] [Google Scholar]
- Long S. R., Staskawicz B. J. Prokaryotic plant parasites. Cell. 1993 Jun 4;73(5):921–935. doi: 10.1016/0092-8674(93)90271-q. [DOI] [PubMed] [Google Scholar]
- Loubens I., Debarbieux L., Bohin A., Lacroix J. M., Bohin J. P. Homology between a genetic locus (mdoA) involved in the osmoregulated biosynthesis of periplasmic glucans in Escherichia coli and a genetic locus (hrpM) controlling pathogenicity of Pseudomonas syringae. Mol Microbiol. 1993 Oct;10(2):329–340. doi: 10.1111/j.1365-2958.1993.tb01959.x. [DOI] [PubMed] [Google Scholar]
- Miller K. J., Gore R. S., Benesi A. J. Phosphoglycerol substituents present on the cyclic beta-1,2-glucans of Rhizobium meliloti 1021 are derived from phosphatidylglycerol. J Bacteriol. 1988 Oct;170(10):4569–4575. doi: 10.1128/jb.170.10.4569-4575.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. J., Gore R. S., Johnson R., Benesi A. J., Reinhold V. N. Cell-associated oligosaccharides of Bradyrhizobium spp. J Bacteriol. 1990 Jan;172(1):136–142. doi: 10.1128/jb.172.1.136-142.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. J., Kennedy E. P., Reinhold V. N. Osmotic adaptation by gram-negative bacteria: possible role for periplasmic oligosaccharides. Science. 1986 Jan 3;231(4733):48–51. doi: 10.1126/science.3941890. [DOI] [PubMed] [Google Scholar]
- Miller K. J., Reinhold V. N., Weissborn A. C., Kennedy E. P. Cyclic glucans produced by Agrobacterium tumefaciens are substituted with sn-1-phosphoglycerol residues. Biochim Biophys Acta. 1987 Jul 10;901(1):112–118. doi: 10.1016/0005-2736(87)90262-8. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay P., Williams J., Mills D. Molecular analysis of a pathogenicity locus in Pseudomonas syringae pv. syringae. J Bacteriol. 1988 Dec;170(12):5479–5488. doi: 10.1128/jb.170.12.5479-5488.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parente J. P., Cardon P., Leroy Y., Montreuil J., Fournet B., Ricart G. A convenient method for methylation of glycoprotein glycans in small amounts by using lithium methylsulfinyl carbanion. Carbohydr Res. 1985 Aug 15;141(1):41–47. doi: 10.1016/s0008-6215(00)90753-5. [DOI] [PubMed] [Google Scholar]
- Puvanesarajah V., Schell F. M., Stacey G., Douglas C. J., Nester E. W. Role for 2-linked-beta-D-glucan in the virulence of Agrobacterium tumefaciens. J Bacteriol. 1985 Oct;164(1):102–106. doi: 10.1128/jb.164.1.102-106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolin D. B., Pfeffer P. E., Osman S. F., Szwergold B. S., Kappler F., Benesi A. J. Structural studies of a phosphocholine substituted beta-(1,3);(1,6) macrocyclic glucan from Bradyrhizobium japonicum USDA 110. Biochim Biophys Acta. 1992 Jun 12;1116(3):215–225. doi: 10.1016/0304-4165(92)90014-l. [DOI] [PubMed] [Google Scholar]
- Schneider J. E., Reinhold V., Rumley M. K., Kennedy E. P. Structural studies of the membrane-derived oligosaccharides of Escherichia coli. J Biol Chem. 1979 Oct 25;254(20):10135–10138. [PubMed] [Google Scholar]
- Schulman H., Kennedy E. P. Localization of membrane-derived oligosaccharides in the outer envelope of Escherichia coli and their occurrence in other Gram-negative bacteria. J Bacteriol. 1979 Jan;137(1):686–688. doi: 10.1128/jb.137.1.686-688.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- van Golde L. M. Metabolism of membrane phospholipids and its relation to a novel class of oligosaccharides in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1368–1372. doi: 10.1073/pnas.70.5.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]


