Abstract
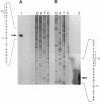
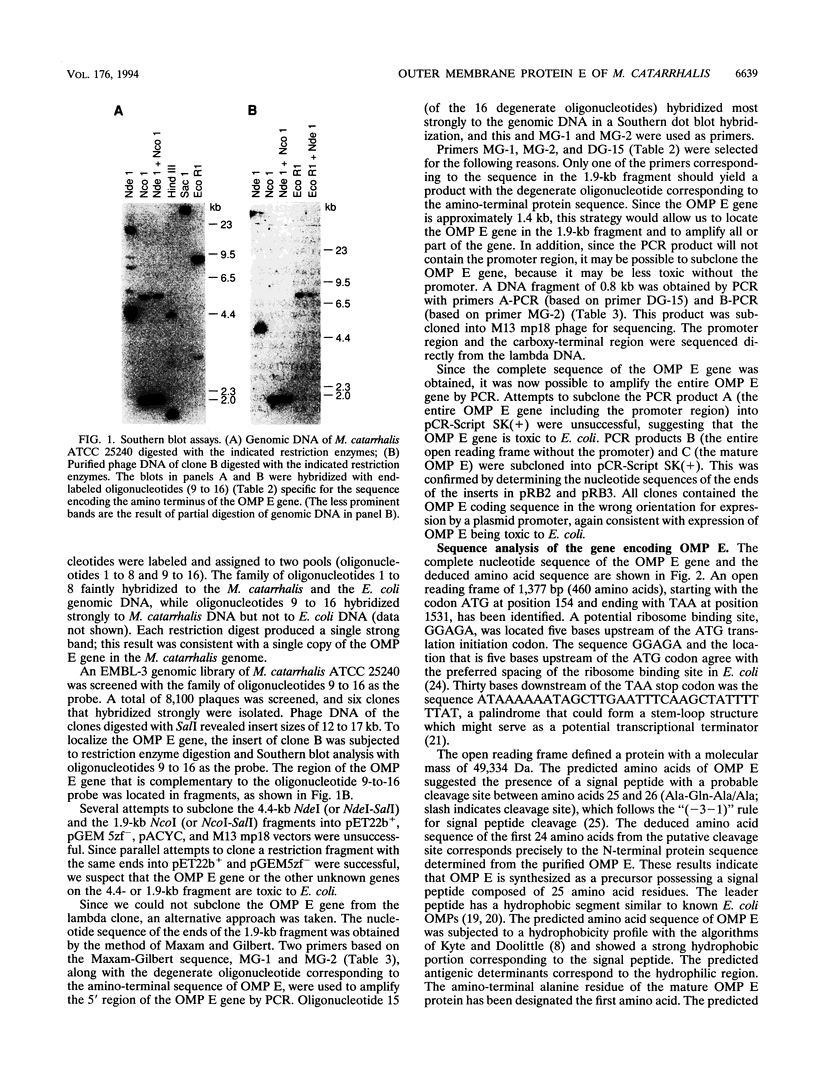
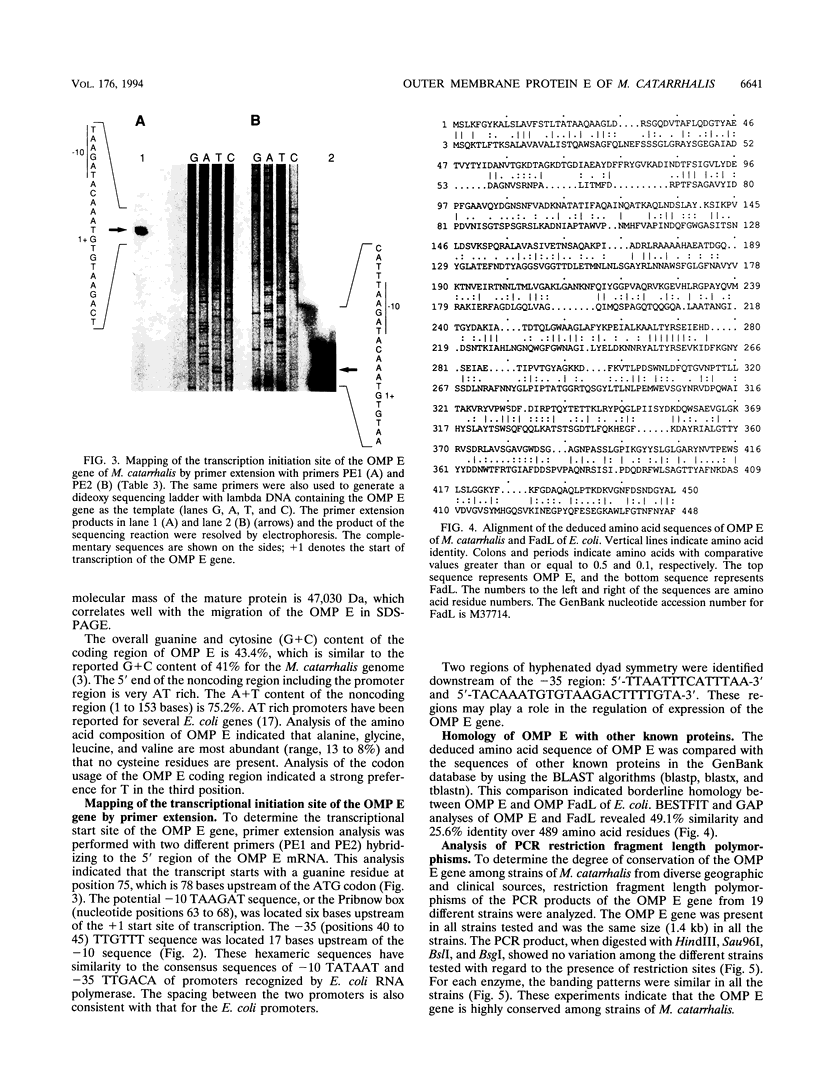
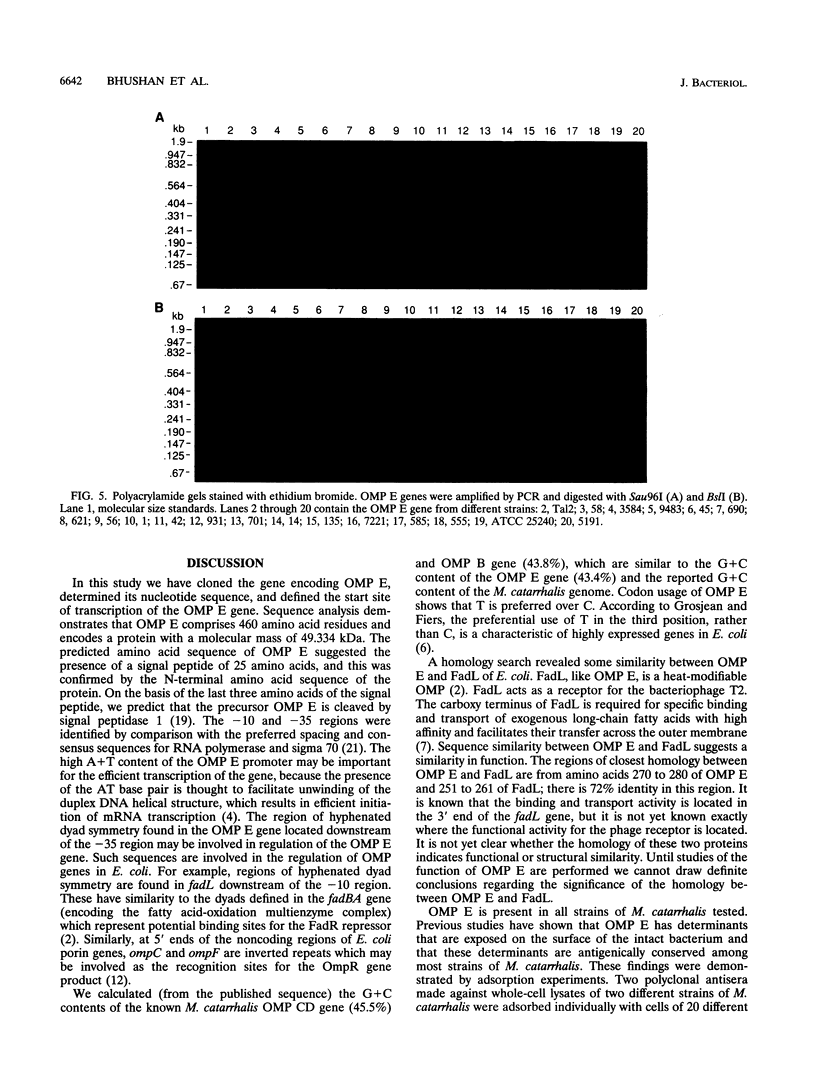
Outer membrane protein E (OMP E) is a 50-kDa protein of Moraxella (Branhamella) catarrhalis. It is a potential vaccine antigen because it is expressed on the surface of the bacterium and has antigenic determinants which are conserved among most strains of M. catarrhalis. To clone the gene encoding OMP E, an EMBL-3 genomic library of strain 25240 was screened with a family of degenerate oligonucleotides based on the amino-terminal protein sequence. The OMP E gene was identified in one of the six positive clones by Southern blot analysis. An open reading frame of 1,377 bp encoding a protein of 460 amino acids was identified. The calculated molecular mass of the mature protein of 436 amino acid residues was 47.03 kDa, which correlated well with the results of sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The protein product of the OMP E gene had a leader peptide of 25 amino acids and a signal peptidase 1 cleavage site similar to those of known OMPs of Escherichia coli. The transcription initiation site of the OMP E gene was mapped by primer extension to be 78 nucleotides upstream of the ATG start codon. Borderline homology was found to the FadL protein of E. coli (49.1% similarity and 25.6% identity), which is involved in the binding and transport of fatty acids. Analysis of restriction fragment length polymorphisms of the OMP E genes of 19 different strains of M. catarrhalis showed that the OMP E gene is highly conserved. The high degree of conservation of sequences of the OMP E genes of M. catarrhalis from diverse sources, along with earlier observations that the protein contains antigenic determinants on the bacterial surface, indicates that OMP E should be studied further as a potential vaccine antigen.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Black P. N. Primary sequence of the Escherichia coli fadL gene encoding an outer membrane protein required for long-chain fatty acid transport. J Bacteriol. 1991 Jan;173(2):435–442. doi: 10.1128/jb.173.2.435-442.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W. Branhamella catarrhalis: an organism gaining respect as a pathogen. Clin Microbiol Rev. 1990 Oct;3(4):293–320. doi: 10.1128/cmr.3.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Kumar G. B., Black P. N. Bacterial long-chain fatty acid transport. Identification of amino acid residues within the outer membrane protein FadL required for activity. J Biol Chem. 1993 Jul 25;268(21):15469–15476. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Marck C. 'DNA Strider': a 'C' program for the fast analysis of DNA and protein sequences on the Apple Macintosh family of computers. Nucleic Acids Res. 1988 Mar 11;16(5):1829–1836. doi: 10.1093/nar/16.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A comparative study on the genes for three porins of the Escherichia coli outer membrane. DNA sequence of the osmoregulated ompC gene. J Biol Chem. 1983 Jun 10;258(11):6932–6940. [PubMed] [Google Scholar]
- Murphy T. F., Bartos L. C. Surface-exposed and antigenically conserved determinants of outer membrane proteins of Branhamella catarrhalis. Infect Immun. 1989 Oct;57(10):2938–2941. doi: 10.1128/iai.57.10.2938-2941.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Kirkham C., Lesse A. J. The major heat-modifiable outer membrane protein CD is highly conserved among strains of Branhamella catarrhalis. Mol Microbiol. 1993 Oct;10(1):87–97. doi: 10.1111/j.1365-2958.1993.tb00906.x. [DOI] [PubMed] [Google Scholar]
- Murphy T. F. Studies of the outer membrane proteins of Branhamella catarrhalis. Am J Med. 1990 May 14;88(5A):41S–45S. doi: 10.1016/0002-9343(90)90261-b. [DOI] [PubMed] [Google Scholar]
- Murphy T. F. The surface of Branhamella catarrhalis: a systematic approach to the surface antigens of an emerging pathogen. Pediatr Infect Dis J. 1989 Jan;8(1 Suppl):S75–S77. [PubMed] [Google Scholar]
- Nakamura K., Inouye M. DNA sequence of the gene for the outer membrane lipoprotein of E. coli: an extremely AT-rich promoter. Cell. 1979 Dec;18(4):1109–1117. doi: 10.1016/0092-8674(79)90224-1. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Mizushima S. Effects of heating in dodecyl sulfate solution on the conformation and electrophoretic mobility of isolated major outer membrane proteins from Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1411–1422. doi: 10.1093/oxfordjournals.jbchem.a131414. [DOI] [PubMed] [Google Scholar]
- Oliver D. Protein secretion in Escherichia coli. Annu Rev Microbiol. 1985;39:615–648. doi: 10.1146/annurev.mi.39.100185.003151. [DOI] [PubMed] [Google Scholar]
- Richet E., Abcarian P., Nash H. A. The interaction of recombination proteins with supercoiled DNA: defining the role of supercoiling in lambda integrative recombination. Cell. 1986 Sep 26;46(7):1011–1021. doi: 10.1016/0092-8674(86)90700-2. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- von Heijne G. How signal sequences maintain cleavage specificity. J Mol Biol. 1984 Feb 25;173(2):243–251. doi: 10.1016/0022-2836(84)90192-x. [DOI] [PubMed] [Google Scholar]