Abstract
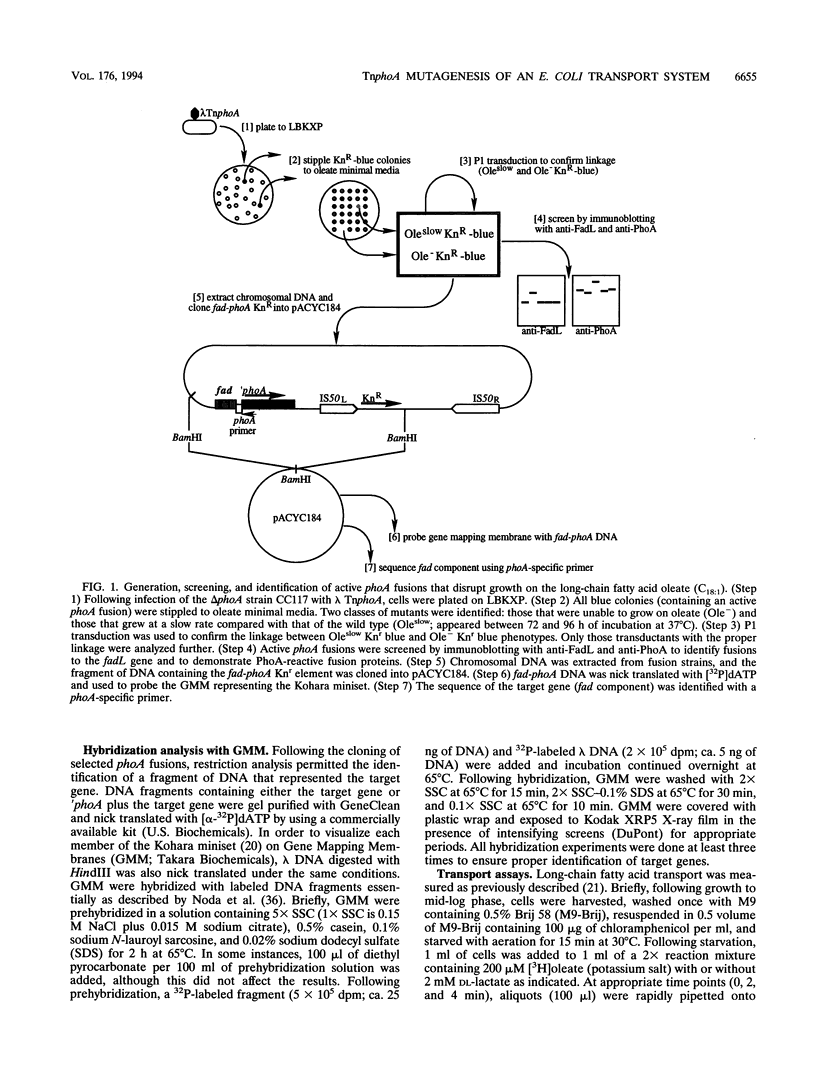
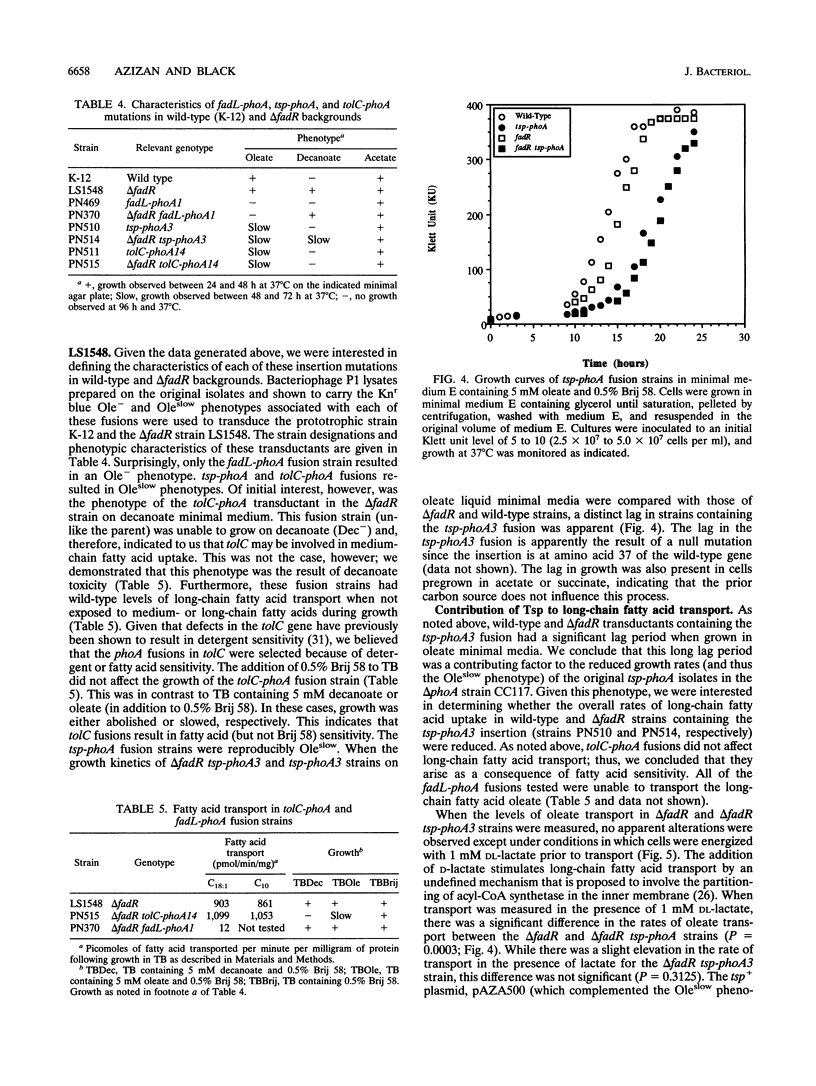
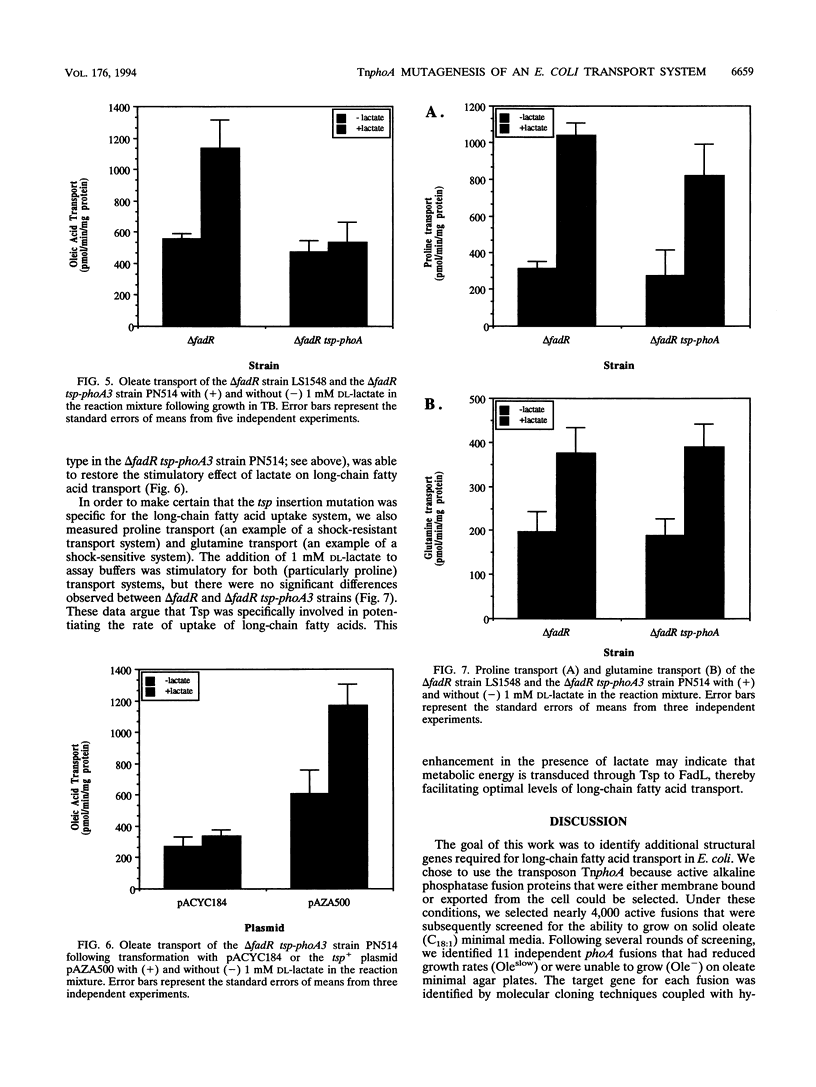
TnphoA was used to mutagenize the chromosome in an effort to identify membrane-bound and exported components of the long-chain fatty acid transport system of Escherichia coli. This strategy identified three classes of fusions that were unable to grow or grew at reduced rates on minimal agar plates containing the long-chain fatty acid oleate (C18:1), (i) fadL-phoA, (ii) tolC-phoA, and (iii) tsp-phoA, fadL-phoA and tolC-phoA fusions were unable to grow on oleate as the sole carbon and energy source, while the tsp-phoA fusion had a markedly reduced growth rate. As expected, fadL-phoA fusions were unable to grow on oleate plates because the outer membrane-bound fatty acid transport protein FadL was defective. The identification of multiple fadL-phoa fusions demonstrated that this strategy of mutagenesis specifically targeted membrane-bound and exported components required for growth on long-chain fatty acids. tolC-phoA fusions were sensitive to fatty acids (particularly medium chain) and thus unable to grow, whereas the reduced growth rate of tsp-phoA fusions on oleate was apparently due to changes in the energized state of the outer membrane or inner membrane. tsp-phoA fusions transported the long-chain fatty acid oleate at only 50% of wild-type levels when cells were energized with 1 mM DL-lactate. Under conditions in which transport was measured in the absence of lactate, tsp-phoA fusion strains and wild-type strains had the same levels of oleate transport. The tsp+ clone pAZA500 was able to restore wild-type transport activity to the tsp-phoA strain under lactate-energized conditions. These results indicate that the periplasmic protein Tsp potentiates long-chain fatty acid transport.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger E. A. Different mechanisms of energy coupling for the active transport of proline and glutamine in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1514–1518. doi: 10.1073/pnas.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black P. N. Characterization of FadL-specific fatty acid binding in Escherichia coli. Biochim Biophys Acta. 1990 Aug 28;1046(1):97–105. doi: 10.1016/0005-2760(90)90099-j. [DOI] [PubMed] [Google Scholar]
- Black P. N., DiRusso C. C., Metzger A. K., Heimert T. L. Cloning, sequencing, and expression of the fadD gene of Escherichia coli encoding acyl coenzyme A synthetase. J Biol Chem. 1992 Dec 15;267(35):25513–25520. [PubMed] [Google Scholar]
- Black P. N., DiRusso C. C. Molecular and biochemical analyses of fatty acid transport, metabolism, and gene regulation in Escherichia coli. Biochim Biophys Acta. 1994 Jan 3;1210(2):123–145. doi: 10.1016/0005-2760(94)90113-9. [DOI] [PubMed] [Google Scholar]
- Black P. N., Kianian S. F., DiRusso C. C., Nunn W. D. Long-chain fatty acid transport in Escherichia coli. Cloning, mapping, and expression of the fadL gene. J Biol Chem. 1985 Feb 10;260(3):1780–1789. [PubMed] [Google Scholar]
- Black P. N. Primary sequence of the Escherichia coli fadL gene encoding an outer membrane protein required for long-chain fatty acid transport. J Bacteriol. 1991 Jan;173(2):435–442. doi: 10.1128/jb.173.2.435-442.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black P. N., Said B., Ghosn C. R., Beach J. V., Nunn W. D. Purification and characterization of an outer membrane-bound protein involved in long-chain fatty acid transport in Escherichia coli. J Biol Chem. 1987 Jan 25;262(3):1412–1419. [PubMed] [Google Scholar]
- Chang C. N., Kuang W. J., Chen E. Y. Nucleotide sequence of the alkaline phosphatase gene of Escherichia coli. Gene. 1986;44(1):121–125. doi: 10.1016/0378-1119(86)90050-8. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot P. H., Scholte H. R., Hülsmann W. C. Fatty acid activation: specificity, localization, and function. Adv Lipid Res. 1976;14:75–126. doi: 10.1016/b978-0-12-024914-5.50009-7. [DOI] [PubMed] [Google Scholar]
- Gutierrez C., Barondess J., Manoil C., Beckwith J. The use of transposon TnphoA to detect genes for cell envelope proteins subject to a common regulatory stimulus. Analysis of osmotically regulated genes in Escherichia coli. J Mol Biol. 1987 May 20;195(2):289–297. doi: 10.1016/0022-2836(87)90650-4. [DOI] [PubMed] [Google Scholar]
- Hara H., Yamamoto Y., Higashitani A., Suzuki H., Nishimura Y. Cloning, mapping, and characterization of the Escherichia coli prc gene, which is involved in C-terminal processing of penicillin-binding protein 3. J Bacteriol. 1991 Aug;173(15):4799–4813. doi: 10.1128/jb.173.15.4799-4813.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameda K., Nunn W. D. Purification and characterization of acyl coenzyme A synthetase from Escherichia coli. J Biol Chem. 1981 Jun 10;256(11):5702–5707. [PubMed] [Google Scholar]
- Kameda K. Partial purification and characterization of fatty acid binding protein(s) in Escherichia coli membranes and reconstitution of fatty acid transport system. Biochem Int. 1986 Aug;13(2):343–350. [PubMed] [Google Scholar]
- Kameda K., Suzuki L. K., Imai Y. Further purification, characterization and salt activation of acyl-CoA synthetase from Escherichia coli. Biochim Biophys Acta. 1985 May 29;840(1):29–36. doi: 10.1016/0304-4165(85)90158-8. [DOI] [PubMed] [Google Scholar]
- Kameda K., Suzuki L. K., Imai Y. Transport of fatty acid is obligatory coupled with H+ entry in spheroplasts of Escherichia coli K12. Biochem Int. 1987 Feb;14(2):227–234. [PubMed] [Google Scholar]
- Klein K., Steinberg R., Fiethen B., Overath P. Fatty acid degradation in Escherichia coli. An inducible system for the uptake of fatty acids and further characterization of old mutants. Eur J Biochem. 1971 Apr;19(3):442–450. doi: 10.1111/j.1432-1033.1971.tb01334.x. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Kumar G. B., Black P. N. Bacterial long-chain fatty acid transport. Identification of amino acid residues within the outer membrane protein FadL required for activity. J Biol Chem. 1993 Jul 25;268(21):15469–15476. [PubMed] [Google Scholar]
- Kumar G. B., Black P. N. Linker mutagenesis of a bacterial fatty acid transport protein. Identification of domains with functional importance. J Biol Chem. 1991 Jan 15;266(2):1348–1353. [PubMed] [Google Scholar]
- Lewis K. Multidrug resistance pumps in bacteria: variations on a theme. Trends Biochem Sci. 1994 Mar;19(3):119–123. doi: 10.1016/0968-0004(94)90204-6. [DOI] [PubMed] [Google Scholar]
- Liou G. I., Geng L., Baehr W. Interphotoreceptor retinoid-binding protein: biochemistry and molecular biology. Prog Clin Biol Res. 1991;362:115–137. [PubMed] [Google Scholar]
- Mangroo D., Gerber G. E. Fatty acid uptake in Escherichia coli: regulation by recruitment of fatty acyl-CoA synthetase to the plasma membrane. Biochem Cell Biol. 1993 Jan-Feb;71(1-2):51–56. doi: 10.1139/o93-008. [DOI] [PubMed] [Google Scholar]
- Mangroo D., Gerber G. E. Photoaffinity labeling of fatty acid-binding proteins involved in long chain fatty acid transport in Escherichia coli. J Biol Chem. 1992 Aug 25;267(24):17095–17101. [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Mekalanos J. J., Beckwith J. Alkaline phosphatase fusions: sensors of subcellular location. J Bacteriol. 1990 Feb;172(2):515–518. doi: 10.1128/jb.172.2.515-518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morona R., Manning P. A., Reeves P. Identification and characterization of the TolC protein, an outer membrane protein from Escherichia coli. J Bacteriol. 1983 Feb;153(2):693–699. doi: 10.1128/jb.153.2.693-699.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morona R., Reeves P. The tolC locus of Escherichia coli affects the expression of three major outer membrane proteins. J Bacteriol. 1982 Jun;150(3):1016–1023. doi: 10.1128/jb.150.3.1016-1023.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Porins and specific channels of bacterial outer membranes. Mol Microbiol. 1992 Feb;6(4):435–442. doi: 10.1111/j.1365-2958.1992.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Saier M. H., Jr Transport proteins in bacteria: common themes in their design. Science. 1992 Nov 6;258(5084):936–942. doi: 10.1126/science.1279804. [DOI] [PubMed] [Google Scholar]
- Niki H., Imamura R., Ogura T., Hiraga S. Nucleotide sequence of the tolC gene of Escherichia coli. Nucleic Acids Res. 1990 Sep 25;18(18):5547–5547. doi: 10.1093/nar/18.18.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda A., Courtright J. B., Denor P. F., Webb G., Kohara Y., Ishihama A. Rapid identification of specific genes in E. coli by hybridization to membranes containing the ordered set of phage clones. Biotechniques. 1991 Apr;10(4):474, 476-7. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu C. W., Freese E. Lipopolysaccharide layer protection of gram-negative bacteria against inhibition by long-chain fatty acids. J Bacteriol. 1973 Sep;115(3):869–875. doi: 10.1128/jb.115.3.869-875.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber K. R., Keiler K. C., Sauer R. T. Tsp: a tail-specific protease that selectively degrades proteins with nonpolar C termini. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):295–299. doi: 10.1073/pnas.89.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C., Delepelaire P. TolC, an Escherichia coli outer membrane protein required for hemolysin secretion. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4776–4780. doi: 10.1073/pnas.87.12.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn F. J., Lupski J. R. The use of transposon Tn5 mutagenesis in the rapid generation of correlated physical and genetic maps of DNA segments cloned into multicopy plasmids--a review. Gene. 1984 Feb;27(2):131–149. doi: 10.1016/0378-1119(84)90135-5. [DOI] [PubMed] [Google Scholar]